Bacteriophage MS2
| Bacteriophage MS2 | |
|---|---|
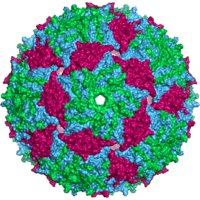 | |
| Bacteriophage MS2 capsid | |
| Virus classification | |
| Group: | Group IV ((+)ssRNA) |
| Family: | Leviviridae |
| Genus: | Levivirus |
| Species: | Bacteriophage MS2 |
The bacteriophage MS2 is an icosahedral, positive-sense single-stranded RNA virus that infects the bacterium Escherichia coli and other members of the Enterobacteriaceae.[1] MS2 is a member of a family of closely related bacterial viruses that includes bacteriophage f2, bacteriophage Qβ, R17, and GA.[2]
History
In 1961, MS2 was isolated by Alvin John Clark and recognized as an RNA-containing phage very similar to bacteriophage f2.[3]
In 1976, the MS2 genome was the first genome to be completely sequenced.[4] This was accomplished by Walter Fiers and his team, building upon their earlier milestone in 1972 of the first gene to be completely sequenced, the MS2 coat protein.[5] These sequences were determined at the RNA level, whereas the next landmark achievement, the sequence of the bacteriophage ΦX174 genome in 1977, was determined using DNA.[6] The first effort at a statistical analysis of the MS2 genome was a search for patterns in the nucleotide sequence. Several non-coding sequences were identified, however at the time of this investigation (1979), the functions of the non-coding patterns were unknown. [7]
Virology
Genome and gene products
The MS2 genome is one of the smallest known, consisting of 3569 nucleotides of single-stranded RNA.[4] It encodes just four proteins: the maturation protein (A-protein), the lysis protein, the coat protein, and the replicase protein.[1] The gene encoding lysis protein (lys) overlaps both the 3'-end of the upstream gene (cp) and the 5'-end of the downstream gene (rep), and was one of the first known examples of overlapping genes. The positive-stranded RNA genome serves as messenger RNA, and is translated upon viral uncoating within the host cell. Although the four proteins are encoded by the same messenger/viral RNA, they are not all expressed at the same levels; expression of these proteins is regulated by a complex interplay between translation and RNA secondary structure.

| Gene | Gene length | Gene product | Amino acids |
|---|---|---|---|
| mat (MS2g1) | 1487 nt | maturation protein | 393 |
| cp (MS2g2) | 510 nt | coat protein | 130 |
| lys (MS2g3) | 295 nt | lysis protein | 75 |
| rep (MS2g4) | 2055 nt | RNA replicase, beta subunit | 545 |
Capsid structure

An MS2 virion (viral particle) is about 27 nm in diameter, as determined by electron microscopy.[8] It consists of one copy of the maturation protein and 180 copies of the coat protein (organized as 90 dimers) arranged into an icosahedral shell with triangulation number T=3, protecting the genomic RNA inside.[9] The virion has an isoelectric point (pI) of 3.9.[10]
The structure of the coat protein is a five-stranded β-sheet with two α-helices and a hairpin. When the capsid is assembled, the helices and hairpin face the exterior of the particle, while the β-sheet faces the interior.[11]
Life cycle
MS2 infects enteric bacteria carrying the fertility (F) factor, a plasmid that allows cells to serve as DNA donors in bacterial conjugation. Genes on the F plasmid lead to the production of an F pilus, which serves as the viral receptor. MS2 attaches to the side of the pilus via its single maturation protein. The precise mechanism by which phage RNA enters the bacterium is unknown.
Once the viral RNA has entered the cell, it begins to function as a messenger RNA for the production of phage proteins. The gene for the most abundant protein, the coat protein, can be immediately translated. The translation start of the replicase gene is normally hidden within RNA secondary structure, but can be transiently opened as ribosomes pass through the coat protein gene. Replicase translation is also shut down once large amounts of coat protein have been made; coat protein dimers bind and stabilize the RNA "operator hairpin", blocking the replicase start. The start of the maturation protein gene is accessible in RNA being replicated but hidden within RNA secondary structure in the completed MS2 RNA; this ensures translation of only a very few copies of maturation protein per RNA. Finally, the lysis protein gene can only be initiated by ribosomes that have completed translation of the coat protein gene and "slip back" to the start of the lysis protein gene, at about a 5% frequency.[1]
Replication of the plus-strand MS2 genome requires synthesis of the complementary minus strand RNA, which can then be used as a template for synthesis of a new plus strand RNA. MS2 replication has been much less well studied than replication of the highly related bacteriophage Qβ, partly because the MS2 replicase has been difficult to isolate, but is likely to be similar.[1]
The formation of the virion is thought to be initiated by binding of maturation protein to the MS2 RNA; in fact, the complex of maturation protein and RNA is infectious. The assembly of the icosahedral shell or capsid from coat proteins can occur in the absence of RNA; however, capsid assembly is nucleated by coat protein dimer binding to the operator hairpin, and assembly occurs at much lower concentrations of coat protein when MS2 RNA is present.[1]
Bacterial lysis and release of newly formed virions occurs when sufficient lysis protein has accumulated. Lysis protein forms pores in the cytoplasmic membrane, which leads to loss of membrane potential and breakdown of the cell wall.[1]
Applications
Since 1998,[12] the MS2 operator hairpin and coat protein have found utility in the detection of RNA in living cells (see MS2 tagging). MS2 and other viral capsids are also currently under investigation as agents in drug delivery, tumor imaging, and light harvesting applications [13]
MS-2, due to its structural similarities to noroviruses, its similar optimum proliferation conditions, and non-pathogenicity to humans, has been used as substitute for noroviruses in studies of disease transmission.[14]
See also
References
- 1 2 3 4 5 6 van Duin, J.; Tsareva, N. (2006). "Single-stranded RNA phages. Chapter 15". In Calendar, R. L. The Bacteriophages (Second ed.). Oxford University Press. pp. 175–196. ISBN 0195148509.
- ↑ Ni, C. Z. et al. Crystal structure of the coat protein from the GA bacteriophage: model of the unassembled dimer. Protein Sci. 5, 2485–2493 (1996)
- ↑ Davis, J. E.; Strauss, J. H.; Sinsheimer, R. L. (1961). "Bacteriophage MS2: another RNA phage". Science. 134 (3488): 1427. doi:10.1126/science.134.3488.1425.
- 1 2 Fiers, W.; Contreras, R.; Duerinck, F; Haegeman, G.; Iserentant, D.; Merregaert, J.; Min Jou, W.; Molemans, F.; Raeymaekers, A.; Van den Berghe, A.; Volckaert, G.; Ysebaert, M. (1976). "Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene". Nature. 260 (5551): 500–507. PMID 1264203. doi:10.1038/260500a0.
- ↑ Min Jou, W.; Haegeman, G.; Ysebaert, M.; Fiers, W. (1972). "Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein". Nature. 237 (5350): 82–88. PMID 4555447. doi:10.1038/237082a0.
- ↑ Sanger, F. F.; et al. (1977). "Nucleotide sequence of bacteriophage φX174 DNA". Nature. 265 (5596): 687–695. PMID 870828. doi:10.1038/265687a0.
- ↑ A search for patterns in the nucleotide sequence of the MS2 genome, Erickson, JW and Altman, GG; J. Mathematical Biology, April 1979, Volume 7, pp219–230
- ↑ Strauss, J. H.; Sinsheimer, R. L. (1963). "Purification and properties of bacteriophage MS2 and of its ribonucleic acid". J Mol Biol. 7 (1): 43–54. doi:10.1016/S0022-2836(63)80017-0.
- ↑ Valegård, K.; Lilias, L.; Fridborg, K.; Unge, T. (1990). "The three-dimensional structure of the bacterial virus MS2". Nature. 345 (6270): 36–41. doi:10.1038/345036a0.
- ↑ Dowd, S. E.; et al. (1998). "Delineating the Specific Influence of Virus Isoelectric Point and Size on Virus Adsorption and Transport Through Sandy Soils". Appl. Environ. Microbiol. 64 (2): 405–410.
- ↑ Golmohammadi, R.; Valegård, K.; Fridborg, K.; Liljas, L. (1993). "The refined structure of bacteriophage MS2 at 2·8 Å resolution". J Mol Biol. 234 (3): 620–639. doi:10.1006/jmbi.1993.1616.
- ↑ Bertrand, E., Chartrand, P., Schaefer, M., Shenoy, S.M., Singer, R.H., and Long, R.M. (1998). Localization of ASH1 mRNA particles in living yeast" Mol. Cell 2, 437–445.
- ↑ Glasgow, J. & Tullman-Ercek, D. Production and applications of engineered viral capsids. Appl. Microbiol. Biotechnol. 98, 5847–58 (2014).
- ↑ Maggie Fox. "Viruses spread 'like crazy' in an office, study finds".
External links
- Complete genome (also isolates R17, DL16, and J20)