Arndt–Eistert reaction
The Arndt–Eistert synthesis is a series of chemical reactions designed to convert a carboxylic acid to a higher carboxylic acid homologue (i.e. contains one additional carbon atom) and is considered a homologation process.[1][2][3] Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), Arndt–Eistert synthesis is a popular method of producing β-amino acids from α-amino acids. Acid chlorides react with diazomethane to give diazoketones. In the presence of a nucleophile (water) and a metal catalyst (Ag2O), diazoketones will form the desired acid homologue.[4][5]
While the classic Arndt–Eistert synthesis uses thionyl chloride to convert the starting acid to an acid chloride, any procedure can be used that will generate an acid chloride.
Diazoketones are typically generated as described here, but other methods such as diazo-group transfer can also apply.[6]
Since diazomethane is toxic and violently explosive, many safer alternatives have been developed,[7] such as the usage of ynolates (Kowalski ester homologation)[8] or diazo(trimethylsilyl)methane.[9][10]
Reaction mechanism
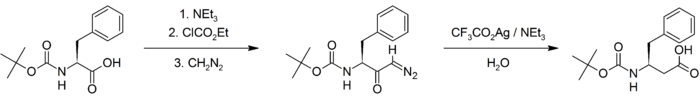
The key step in the Arndt–Eistert synthesis is the metal-catalyzed Wolff rearrangement of the diazoketone to form a ketene.[11] In the insertion homologation of t-BOC protected (S)-phenylalanine (2-amino-3-phenylpropanoic acid), t-BOC protected (S)-3-amino-4-phenylbutanoic acid is formed.[5]
Wolff rearrangement[12][13] of the α-diazoketone intermediate forms a ketene via a 1,2-rearrangement, which is subsequently hydrolysed to form the carboxylic acid. The consequence of the 1,2-rearrangement is that the methylene group alpha to the carboxyl group in the product is the methylene group from the diazomethane reagent. It has been demonstrated that the rearrangement preserves the stereochemistry of the chiral centre as the product formed from t-BOC protected (S)-phenylalanine retains the (S) stereochemistry with a reported enantiomeric excess of at least 99%.[5]
Heat, light, platinum, silver, and copper salts will also catalyze the Wolff rearrangement to produce the desired acid homologue.
Variations
In the Newman–Beal modification, addition of triethylamine to the diazomethane solution will avoid the formation of α-chloromethylketone side-products.[14]
See also
References
- ↑ Arndt, F.; Eistert, B. (1935). "Ein Verfahren zur Überführung von Carbonsäuren in ihre höheren Homologen bzw. deren Derivate". Ber. Dtsch. Chem. Ges. (in German). 68 (1): 200–208. doi:10.1002/cber.19350680142.
- ↑ Bachmann, W. E.; Struve, W. S. (1942). "The Arndt–Eistert Reaction". Org. React.. 1: 38.
- ↑ Ye, T.; McKervey, M. A. (1994). "Organic Synthesis with α-Diazo Carbonyl Compounds". Chem. Rev. 94 (4): 1091–1160. doi:10.1021/cr00028a010.
- ↑ Lee, V.; Newman, M. S. (1970). "Ethyl 1-Naphthylacetate". Org. Synth. 50: 77.; Coll. Vol., 6, p. 613
- 1 2 3 Linder, M. R.; Steurer, S.; Podlech, J. (2002). "(S)-3-(tert-Butyloxycarbonylamino)-4-phenylbutanoic acid". Org. Synth. 79: 154.; Coll. Vol., 10, p. 194
- ↑ Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1996). "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one". Org. Synth. 73: 134.; Coll. Vol., 9, p. 197
- ↑ Katritzky, A. R.; Zhang, S.; Hussein, A. H. M.; Fang, Y.; Steel, P. J. (2001). "One-Carbon Homologation of Carboxylic Acids via BtCH2TMS: A Safe Alternative to the Arndt−Eistert Reaction". J. Org. Chem. 66 (16): 5606–5612. doi:10.1021/jo0017640.
- ↑ Reddy, R. E.; Kowalski, C. J. (1993). "Ethyl 1-Naphthylacetate: Ester Homologation Via Ynolate Anions". Org. Synth. 71: 146.; Coll. Vol., 9, p. 426
- ↑ Aoyama, T.; Shiori, T. (1980). "New Methods and Reagents in Organic Synthesis. 8. Trimethylsilyldiazomethane. A New, Stable, and Safe Reagent for the Classical Arndt-Eistert Synthesis". Tetrahedron Lett. 21 (46): 4461–4462. doi:10.1016/S0040-4039(00)92200-7.
- ↑ Cesar, J.; Dolenc, M. S. (2001). "Trimethylsilyldiazomethane in the Preparation of Diazoketones via Mixed Anhydride and Coupling Reagent Methods: a New Approach to the Arndt–Eistert Synthesis". Tetrahedron Lett. 42 (40): 7099–7102. doi:10.1016/S0040-4039(01)01458-7.
- ↑ Huggett, C.; Arnold, R. T.; Taylor, T. I. (1942). "The Mechanism of the Arndt-Eistert Reaction". J. Amer. Chem. Soc. 64 (12): 3043. doi:10.1021/ja01264a505.
- ↑ Meier, H.; Zeller, K.-P. (1975). "The Wolff Rearrangement of α-Diazo Carbonyl Compounds". Angew. Chem. Int. Ed. 14 (1): 32–43. doi:10.1002/anie.197500321.
- ↑ Kirmse, W. (2002). "100 Years of the Wolff Rearrangement". Eur. J. Org. Chem. 2002 (14): 2193–2256. doi:10.1002/1099-0690(200207)2002:14<2193::AID-EJOC2193>3.0.CO;2-D.
- ↑ Newman, M. S.; Beal, Philip F. (1950). "An Improved Wolff Rearrangement in Homogeneous Medium". J. Amer. Chem. Soc. 72 (11): 5163–5165. doi:10.1021/ja01167a101.