Anastrozole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arimidex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696018 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 83–85% |
| Protein binding | 40% |
| Metabolism | Liver (85%) |
| Biological half-life | 46.8 h [2] |
| Excretion | Kidney (11%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.129.723 |
| Chemical and physical data | |
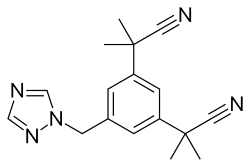
| Formula | C17H19N5 |
| Molar mass | 293.366 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Anastrozole, sold under the trade name Arimidex among others, is a medication used in addition to other treatments for breast cancer. Specifically it is used for hormone receptor-positive breast cancer. It has also been used to prevent breast cancer in those at high risk. It is taken by mouth.[1]
Common side effects include hot flashes, altered mood, joint pain, and nausea. Severe side effects include an increased risk of heart disease and osteoporosis. Use during pregnancy is known to harm the baby. Anastrozole is in the aromatase-inhibiting family of medications. It works by blocking the creation of estrogen.[1]
Anastrozole was patented in 1987 and approved for medical use in 1995.[4] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[5] Anastrozole is available as a generic medication.[1] The wholesale cost in the developing world is about 1.92 to 30.60 USD a month.[6] In the United States the wholesale cost is about 3.81 USD per month.[7]
Medical uses

The ATAC trial was of localized breast cancer and women received either anastrozole, tamoxifen, or both for five years, followed by five years of follow-up.[8] After more than 5 years the group that received anastrozole had better results than the tamoxifen group.[8] The trial suggested that anastrozole is the preferred medical therapy for postmenopausal women with localized breast cancer, which is estrogen receptor (ER) positive.[8] Another study found that the risk of recurrence was reduced by 40%, but was associated with an increased risk of bone fractures. The study concluded that ER positive patients benefited from switching from tamoxifen to anastrozole in patients who have completed 2 years' adjuvant tamoxifen.[9] A more recent trial found that anastrozole significantly reduced the incidence of breast cancer in postmenopausal women relative to placebo, and while there were side effects related to estrogen deprivation observed, the researchers concluded that this was probably not related to the treatment. Lead author Jack Cuzick was quoted by the BBC as saying, "This class of drugs is more effective than previous drugs such as tamoxifen and crucially, it has fewer side effects," adding that he thought there was now enough evidence to support offering the drug.[10]
Side effects
Bone weakness has been associated with anastrozole. Women who switched to anastrozole after two years on tamoxifen reported twice as many fractures as those who continued to take tamoxifen (2.1% compared to 1%).[9] Bisphosphonates are sometimes prescribed to prevent the osteoporosis induced by aromatase inhibitors. The level of circulating estradiol is likely causal here and not the anastrozole itself, and so the dose will determine likelihood of osteoporosis (estradiol inhibits osteoclasts, which resorb bone). Acne, constricted pupils and water retention have also been attributed with use of this anti-estrogen.
Pharmacology
Anastrozole reversibly binds to the aromatase enzyme, and through competitive inhibition blocks the conversion of androgens to estrogens in peripheral (extragonadal) tissues.[11]
Pharmacodynamics
Anastrozole is poorly able to cross the blood–brain barrier into the central nervous system.[12] This is due to P-glycoprotein-mediated efflux.[12]
Research
Usage in men
Anastrozole has been tested for reducing estrogens, including estradiol, in men.[13] Excess estradiol in men can cause benign prostatic hyperplasia, gynecomastia, and symptoms of hypogonadism. It can also contribute to increased risk of stroke, heart attack, chronic inflammation, prostate enlargement and prostate cancer.[14] Some athletes and body builders use anastrozole as part of their steroid cycle to reduce and prevent symptoms of excess estrogen--gynecomastia, emotional lability and water retention.[13] Study data suggest dosages of 0.5 mg to 1 mg a day reduce serum estradiol by approximately 50% in men, which differs in postmenopausal women.[15]
Usage in children
Anastrozole may be used off-label in children with precocious puberty, or children with pubertal gynecomastia.[2][16] Following the onset of puberty, the epiphyseal plate begins to close due to an increased amount of estrogen production escaping local metabolism and spreading to the circulatory system.[16] It is shown to help slow this process, and increase adult height prediction in adolescent males treated with protein-based peptide hormones for the treatment of growth hormone deficiency.[17]
References
- 1 2 3 4 "Anastrozole". The American Society of Health-System Pharmacists. Retrieved 8 December 2016.
- 1 2 Mauras N, Bishop K, Merinbaum D, Emeribe U, Agbo F, Lowe E (August 2009). "Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent-onset gynecomastia". J. Clin. Endocrinol. Metab. 94 (8): 2975–8. PMID 19470631. doi:10.1210/jc.2008-2527.
- ↑ "anastrozole". Chemical Entities of Biological Interest (ChEBI). European Molecular Biology Laboratory. Retrieved 2011-08-14.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 516. ISBN 9783527607495.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- ↑ "Anastrozole". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ↑ "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Retrieved 18 December 2016.
- 1 2 3 Howell A; Cuzick J; Baum M; et al. (2005). "Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer". Lancet. 365 (9453): 60–2. PMID 15639680. doi:10.1016/S0140-6736(04)17666-6.
- 1 2 Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J (2005). "Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial". Lancet. 366 (9484): 455–62. PMID 16084253. doi:10.1016/S0140-6736(05)67059-6.
- ↑ Gallagher, James (12 December 2013). "Breast cancer: Drug 'halves' risk of tumours". BBC News. Retrieved 12 December 2013.
- ↑ Simpson ER (September 2003). "Sources of estrogen and their importance". J. Steroid Biochem. Mol. Biol. 86 (3–5): 225–30. PMID 14623515. doi:10.1016/S0960-0760(03)00360-1.
- 1 2 Russell N, Cheung A, Grossmann M (2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. PMID 28667081. doi:10.1530/ERC-17-0153.
- 1 2 Mauras N, O'Brien KO, Klein KO, Hayes V (July 2000). "Estrogen suppression in males: metabolic effects". J. Clin. Endocrinol. Metab. 85 (7): 2370–7. PMID 10902781. doi:10.1210/jc.85.7.2370.; Dougherty RH, Rohrer JL, Hayden D, Rubin SD, Leder BZ (February 2005). "Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels". Clin. Endocrinol. (Oxf). 62 (2): 228–35. PMID 15670201. doi:10.1111/j.1365-2265.2005.02205.x.
- ↑ Faloon, William. "Dangers of Excess Estrogen In the Aging Male". Life Extension Magazine, November 2008.
- ↑ Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C (March 2004). "Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels". J. Clin. Endocrinol. Metab. 89 (3): 1174–80. PMID 15001605. doi:10.1210/jc.2003-031467.
- 1 2 Faglia G, Arosio M, Porretti S (December 2000). "Delayed closure of epiphyseal cartilages induced by the aromatase inhibitor anastrozole. Would it help short children grow up?". J. Endocrinol. Invest. 23 (11): 721–3. PMID 11194703. doi:10.1007/bf03345059.
- ↑ Mauras N, Gonzalez de Pijem L, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID, Klein KO, Singh RJ, Miyamoto A, Bishop K (March 2008). "Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a randomized, placebo-controlled, multicenter trial for one to three years". J. Clin. Endocrinol. Metab. 93 (3): 823–31. PMC 2266949
 . PMID 18165285. doi:10.1210/jc.2007-1559.
. PMID 18165285. doi:10.1210/jc.2007-1559.