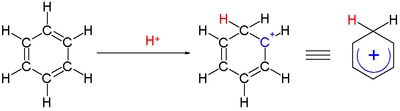
Arenium ion

An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.[1] For historic reasons this complex is also called a Wheland intermediate[2] or a sigma complex or σ-complex. The smallest arenium ion is the benzenium ion (C6H7+), which is protonated benzene.
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring.[3] The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 3 carbon atoms via the pi system, as depicted on the following resonance structures:
A complexed electrophile can contribute to the stability of arenium ions.
A benzenium ion can be isolated as a stable compound when benzene is protonated by the carborane superacid H(CB11H(CH3)5Br6).[4] The benzenium salt is crystalline with thermal stability up to 150 °C. Bond lengths deduced from X-ray crystallography are consistent with a cyclohexadienyl cation structure.
In one study a methylene arenium ion is stabilized by metal complexation:[5]
In this reaction sequence the R-Pd(II)-Br starting complex 1 stabilized by TMEDA is converted by dppe to metal complex 2. Electrophilic attack of methyl triflate forms methylene arenium ion 3 with (based on X-ray crystallography) positive charge located in aromatic para position and with the methylene group 6° out of the plane of the ring. Reaction first with water and then with triethylamine hydrolyzes the ether group.
See also
References
- ↑ Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions George A. Olah J. Am. Chem. Soc.; 1972; 94(3) pp 808 - 820; doi:10.1021/ja00758a020
- ↑ A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules G. W. Wheland J. Am. Chem. Soc.; 1942; 64(4) pp 900 - 908; doi:10.1021/ja01256a047
- ↑ A guidebook to mechanism in organic chemistry, Peter Sykes; pp 130-133
- ↑ Isolating Benzenium Ion Salts Christopher A. Reed, Kee-Chan Kim, Evgenii S. Stoyanov, Daniel Stasko, Fook S. Tham, Leonard J. Mueller, and Peter D. W. Boyd J. Am. Chem. Soc.; 2003; 125(7) pp 1796 - 1804; doi:10.1021/ja027336o
- ↑ Synthesis and Reactivity of the Methylene Arenium Form of a Benzyl Cation, Stabilized by Complexation Elena Poverenov, Gregory Leitus, and David Milstein J. Am. Chem. Soc.; 2006; 128(51) pp 16450 - 16451; (Communication) doi:10.1021/ja067298z