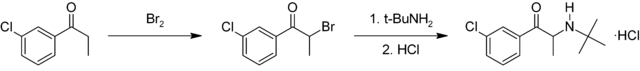Bupropion
 | |
|
1 : 1 mixture (racemate) | |
| Clinical data | |
|---|---|
| Pronunciation |
/bjuːˈproʊ.pi.ɒn/ bew-PROH-pee-on |
| Trade names | Wellbutrin, Elontril, Zyban |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695033 |
| License data | |
| Pregnancy category | |
| Dependence liability | None to very low |
| Addiction liability | None to very low |
| Routes of administration |
Medical: by mouth Recreational: by mouth, insufflation, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 84% (bupropion), 77% (hydroxybupropion metabolite), 42% (threohydrobupropion metabolite)[1] |
| Metabolism | Liver (mostly CYP2B6-mediated hydroxylation, but with some contributions from CYP1A2, CYP2A6, CYP2C9, CYP3A4, CYP2E1 and CYP2C19)[1][2][3][4] |
| Biological half-life | 12–30 hours[3][5] |
| Excretion | Renal (87%; 0.5% unchanged), faecal (10%)[1][2][3] |
| Identifiers | |
| |
| Synonyms |
Amfebutamone; 3-Chloro-N-tert-butyl-β-keto-α-methylphenethylamine; 3-Chloro-N-tert-butylcathinone; Bupropion hydrochloride[6] |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H18ClNO |
| Molar mass | 239.74 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Bupropion is a medication primarily used as an antidepressant and smoking cessation aid.[7][8][9] It is marketed as Wellbutrin and Zyban among other trade names. It is one of the most frequently prescribed antidepressants in the United States and Canada,[10] although in many countries this is an off-label use.[11] It is an effective antidepressant on its own, but is also popular as an add-on medication in cases of incomplete response to first-line SSRI antidepressants.[12] Bupropion is taken in tablet form and is available only by prescription in most countries.[10]
The most important side effect is an increase in risk for epileptic seizures, which caused the drug to be withdrawn from the market for some time and then caused the recommended dosage to be reduced.[12] In comparison to many other antidepressants, it does not cause as much weight gain, sexual dysfunction, or sleepiness.[12] Bupropion acts as an norepinephrine-dopamine reuptake inhibitor (NDRI). It is an atypical antidepressant, different from most commonly prescribed antidepressants such as selective serotonin reuptake inhibitors (SSRIs).[11]
Bupropion is known to affect several different biological targets.[12][13] It often is described as a norepinephrine-dopamine reuptake inhibitor (NDRI), and is also a nicotinic antagonist.[13][14] However, bupropion does not appear to have significant dopaminergic actions in humans under normal clinical circumstances.[13][15] Chemically, bupropion belongs to the class of aminoketones and is similar in structure to stimulants such as cathinone and amfepramone, and to phenethylamines in general.[12]
Bupropion was first made by Nariman Mehta and patented by Burroughs Wellcome in 1969, which later became part of what is now GlaxoSmithKline. It was first approved for medical use in the United States in 1989. It was originally called by the generic name amfebutamone, before being renamed in 2000.[16]
Medical uses

Depression
Bupropion is one of the most widely prescribed antidepressants, and the available evidence indicates that it is effective in clinical depression[17] — as effective as several other widely prescribed drugs, including fluoxetine (Prozac) and paroxetine (Paxil),[18] although trends favoring the efficacy of escitalopram (Lexapro), sertraline (Zoloft) and venlafaxine (Effexor) over bupropion have been observed.[18] Mirtazapine (Remeron), on the other hand is significantly more effective than bupropion.[18] Bupropion has several features that distinguish it from other antidepressants: for instance, unlike the majority of antidepressants, it does not usually cause sexual dysfunction.[19] Bupropion treatment also is not associated with the sleepiness or weight gain that may be produced by other antidepressants.[20]
In depressed people who experience symptoms of sleepiness and fatigue, bupropion has been found to be more effective than selective serotonin reuptake inhibitors (SSRIs) in alleviating these symptoms.[21] There appears to be a modest advantage for the SSRIs over bupropion in the treatment of anxious depression.[22]
According to surveys, the addition to a prescribed SSRI is a common strategy when people do not respond to the SSRI, even though this is not an officially approved indication.[23] The addition of bupropion to an SSRI (most commonly fluoxetine or sertraline) may result in an improvement in some people who have an incomplete response to the first-line antidepressant.[23]
Bupropion was approved by the U.S. Food and Drug Administration (FDA), in 2006, for the prevention of seasonal affective disorder.[24] In some countries (including Australia, New Zealand and the UK) this is an off-label use.[25][26]
Smoking cessation
The next most common use is as an aid for smoking cessation.[27][28] Bupropion reduces the severity of nicotine cravings and withdrawal symptoms.[29] Bupropion is helpful for smoking cessation in smokers with no history of depression; thus, the effectiveness of bupropion is not due to its antidepressant effect. A typical bupropion treatment course lasts up to twelve weeks, with the people halting the use of tobacco about ten days into the course. Bupropion approximately doubles the chance of quitting smoking successfully.[29] The effectiveness of bupropion is comparable to nicotine replacement therapy, but less effective than varenicline.[29]
Animal studies indicate that administration of bupropion at less than the recommended therapeutic dose may actually enhance the rewarding properties of nicotine, i.e., low doses augment nicotine self-administration and high doses attenuate it.[30]
In Australia and the UK smoking cessation is the only licensed use of bupropion.[25][26]
Attention deficit hyperactivity disorder
Bupropion has been used as a treatment for attention deficit hyperactivity disorder (ADHD) since at least 2004,[31] with reports of positive results in both minors and adults.[32] In a double-blind study of children, while aggression and hyperactivity as rated by the children's teachers were significantly improved in comparison to placebo, parents and clinicians could not distinguish between the effects of bupropion and placebo.[32] The 2007 guideline on the ADHD treatment from American Academy of Child and Adolescent Psychiatry notes that the evidence for bupropion is "far weaker" than for the FDA-approved treatments. Its effect may also be "considerably less than of the approved agents ... Thus it may be prudent for the clinician to recommend a trial of behavior therapy at this point, before moving to these second-line agents."[33] Similarly, the Texas Department of State Health Services guideline recommends considering bupropion or a tricyclic antidepressant as a fourth-line treatment after trying two different stimulants and atomoxetine.[34]
Sexual dysfunction
Bupropion is one of few antidepressants that does not cause sexual dysfunction.[35] A range of studies demonstrate that bupropion not only produces fewer sexual side effects than other antidepressants, but can actually help to alleviate sexual dysfunction.[36] According to a survey of psychiatrists, it is the drug of choice for the treatment of SSRI-induced sexual dysfunction, although this is not an indication approved by the U.S. Food and Drug Administration. There have also been a few studies suggesting that bupropion can improve sexual function in women who are not depressed, if they have hypoactive sexual desire disorder (HSDD).[37]
Obesity
Bupropion, when used for treating obesity over a period of 6 to 12 months, may result in weight loss of 2.7 kg over placebo.[38] This is not much different from the weight loss produced by several other medications, such as sibutramine or orlistat.[38]
It has been studied in combination with naltrexone.[39] Concerns from bupropion include an increase in blood pressure and heart rate.[39] In September 2014, a combination (bupropion/naltrexone) was approved by the US FDA for the treatment of obesity.[40]
Other uses
There has been controversy about whether it is useful to add an antidepressant such as bupropion to a mood stabilizer in people with bipolar depression, but recent reviews have concluded that bupropion in this situation does no significant harm and may sometimes give significant benefit.[41][42]
Bupropion has shown no effectiveness in the treatment of cocaine dependence, but there is weak evidence that it may be useful in treating methamphetamine dependence.[43]
Based on studies indicating that bupropion lowers the level of the inflammatory mediator TNF-alpha, there have been suggestions that it might be useful in treating inflammatory bowel disease or other autoimmune conditions, but very little clinical evidence is available.[44]
Bupropion—like other antidepressants, with the exception of duloxetine (Cymbalta)[45]—is not effective in treating chronic low back pain.[46] It does, however, show some promise in the treatment of neuropathic pain.[47]
Contraindications
The drug label advises that bupropion should not be prescribed to individuals with epilepsy or other conditions that lower the seizure threshold, such as anorexia nervosa, bulimia nervosa, active brain tumors, or concurrent alcohol and/or benzodiazepine use and/or withdrawal. It should be avoided in individuals who are also taking monoamine oxidase inhibitors (MAOIs). When switching from MAOIs to bupropion, it is important to include a washout period of about two weeks between the medications.[3] The label recommends that caution should be exercised when treating people with liver damage, severe kidney disease, and severe hypertension, and in children, adolescents and young adults due to the increased risk of suicidal ideation.[3]
Side effects
Epileptic seizures are the most important adverse effect of bupropion. A high incidence of seizures was responsible for the temporary withdrawal of the drug from the market between 1986 and 1989. The risk of seizure is strongly dose-dependent, but also dependent on the preparation. The sustained-release preparation is associated with a seizure incidence of 0.1% at daily dosages of less than 300 mg of bupropion and 0.4% at 300–400 mg.[48] The immediate release preparation is associated with a seizure incidence of 0.4% for dosages below 450 mg; the incidence climbs to 5% for dosages between 450–600 mg per day.[48] For comparison, the incidence of unprovoked seizure in the general population is 0.07 to 0.09%, and the risk of seizure for a variety of other antidepressants is generally between 0 and 0.6% at recommended dosage levels.[49] Clinical depression itself has been reported to increase the occurrence of seizures, and a study examining FDA clinical trial data has suggested that in most cases, low to moderate doses of antidepressants may not actually increase seizure risk at all.[50] However, this study also found that bupropion and clomipramine were unique among antidepressants in that they were associated with increased incidence of seizures.[50]
The prescribing information notes that hypertension, sometimes severe, was observed in some people taking bupropion, both with and without pre-existing hypertension. The frequency of this adverse effect was under 1% and not significantly higher than found with placebo.[3] A review of the available data carried out in 2008 indicated that bupropion is safe to use in people with a variety of serious cardiac conditions.[51]
In the UK, more than 7,600 reports of suspected adverse reactions were collected in the first two years after bupropion's approval by the Medicines and Healthcare Products Regulatory Agency as part of the Yellow Card Scheme, which monitored side effects. Approximately 540,000 people were treated with bupropion for smoking cessation during that period. The MHRA received 60 reports of "suspected [emphasis MHRA's] adverse reactions to Zyban which had a fatal outcome". The agency concluded that "in the majority of cases the individual's underlying condition may provide an alternative explanation."[52] This is consistent with a large, 9,300-subject safety study that showed that the mortality of smokers taking bupropion is not higher than the natural mortality of smokers of the same age.[53]
Psychiatric
Suicidal thoughts and behaviors are rare in clinical trials, and the FDA requires all antidepressants, including bupropion, to carry a boxed warning stating that antidepressants may increase the risk of suicide in persons younger than 25. This warning is based on a statistical analysis conducted by the FDA which found a 2-fold increase in suicidal thought and behavior in children and adolescents, and 1.5-fold increase in the 18–24 age group.[54] For this analysis the FDA combined the results of 295 trials of 11 antidepressants in order to obtain statistically significant results. Considered in isolation, bupropion was not statistically different from placebo.[54]
Suicidal behavior is less of a concern when bupropion is prescribed for smoking cessation. According to a 2014 Cochrane review, while there is an association with suicide it is unclear if bupropion was the cause.[55] In 2016 the FDA removed the black box warning about psychiatric problems when used for stopping smoking.[56]
In 2009 the FDA issued a health advisory warning that the prescription of bupropion for smoking cessation has been associated with reports about unusual behavior changes, agitation and hostility. Some people, according to the advisory, have become depressed or have had their depression worsen, have had thoughts about suicide or dying, or have attempted suicide.[57] This advisory was based on a review of anti-smoking products that identified 75 reports of "suicidal adverse events" for bupropion over ten years.[58]
Bupropion-induced psychosis may develop in select populations, or worsen a pre-existing psychotic syndrome.[59] Symptoms may include delusions, hallucinations, paranoia, and confusion. In most cases these symptoms can be reduced or eliminated by reducing the dose, ceasing treatment or adding antipsychotic medication.[3][59] However, adding a benzodiazepine to treat psychosis, instead of an antipsychotic, may become a valid alternative according to the model of amphetamine-induced psychosis.[60] Psychotic symptoms are associated with factors such as higher doses of bupropion, a history of bipolar disorder or psychosis, concomitant medications, for example, lithium or benzodiazepines, old age, or substance abuse.[59][61]
In a large-scale study of programs where bupropion was used for smoking cessation or treatment of depression, no withdrawal symptoms were observed.[62] As of 2002 there were two case reports of people experiencing withdrawal symptoms when discontinuing buproprion taken to aid smoking cessation;[63] the prescribing information states that dose tapering is not required when discontinuing treatment for smoking cessation.[1]
Overdose
Bupropion is considered moderately dangerous in overdose.[64][65] For significant overdoses, seizures have been reported in about a third of all cases; other serious effects include hallucinations, loss of consciousness, and abnormal heart rhythms. When bupropion was one of several kinds of pills taken in an overdose, fever, muscle rigidity, muscle damage, hypertension or hypotension, stupor, coma, and respiratory failure have been reported. While most people recover, some people have died, and before they died suffered multiple uncontrolled seizures and heart attacks.[3]
In the majority of childhood exploratory ingestions involving one or two tablets, children show no apparent symptoms.[66]
Interactions
Since bupropion is metabolized to hydroxybupropion by the CYP2B6 enzyme, drug interactions with CYP2B6 inhibitors are possible: this includes medications like paroxetine, sertraline, fluoxetine, diazepam, clopidogrel, and orphenadrine. The expected result is the increase of bupropion and decrease of hydroxybupropion blood concentration. The reverse effect (decrease of bupropion and increase of hydroxybupropion) can be expected with CYP2B6 inducers, such as carbamazepine, clotrimazole, rifampicin, ritonavir, St John's wort, phenobarbital, phenytoin and others.[67] Conversely, because bupropion is itself a strong inhibitor of CYP2D6 (Ki = 21 μM),[1][30] as is its active metabolite, hydroxybupropion (Ki = 13.3 μM), it can slow the clearance of other drugs metabolized by this enzyme.[1][2][3][67] As an example, the ratio of dextromethorphan (a drug that is mainly metabolized by CYP2D6) to its major metabolite dextrorphan increased approximately 35-fold when it was administered to people being treated with 300 mg/day bupropion, indicative of a major drug interaction with a common over-the-counter medicine.[67]
Bupropion lowers the threshold for epileptic seizures,[68] and therefore can potentially interact with other medications that also lower it, such as carbapenems, cholinergic agents, fluoroquinolones, interferons, chloroquine, mefloquine, lindane, theophylline, systemic corticosteroids (e.g., prednisone), and some tricyclic antidepressants (e.g., clomipramine).[3] The prescribing information recommends minimizing the use of alcohol, since in rare cases bupropion reduces alcohol tolerance, and because the excessive use of alcohol may lower the seizure threshold.[3] Also, bupropion should not be taken by individuals undergoing abrupt cessation of alcohol or benzodiazepine use.
Caution should be observed when combining bupropion with a monoamine oxidase inhibitor (MAOI), as it may result in hypertensive crisis.[69]
Pharmacology
| Exposure (concentration over time; bupropion exposure = 100%) and half-life | |||||
| Bupropion | R,R- Hydroxy bupropion |
S,S- Hydroxy bupropion |
Threo- hydro bupropion |
Erythro- hydro bupropion | |
|---|---|---|---|---|---|
| Exposure | 100% | 800% | 160% | 310% | 90% |
| Half-life | 10 h (IR) 17 h (SR) |
21 h | 25 h | 26 h | 26 h |
| Inhibition potency (potency of DA uptake inhibition by bupropion = 100%) | |||||
| DA uptake | 100% | ND | ND | ND | ND |
| NE uptake | 27% | ND | ND | ND | ND |
| 5-HT uptake | 2% | ND | ND | ND | ND |
| α3β4 nicotinic | 53% | 15% | 10% | ND | ND |
| α4β2 nicotinic | 8% | 3% | 29% | ND | ND |
| α1* nicotinic | 12% | 13% | 13% | ND | ND |
| DA: dopamine; NE: norepinephrine; 5-HT: serotonin; ND: no data | |||||
Pharmacodynamics
Based on preclinical research with animal and human proteins, bupropion has been characterized as a weak norepinephrine-dopamine reuptake inhibitor (NDRI).[13] It has also been found to act as a releasing agent of dopamine and norepinephrine (NDRA), similarly to other cathinones.[15][75][76] However, when ingested orally by humans, bupropion is extensively converted in the body into several active metabolites with differing activities and influences on the effects of the drug during first-pass metabolism.[13][15] These metabolites are present in much higher concentrations in the body compared to bupropion itself.[13][15][77] The most important example is the major metabolite of bupropion, hydroxybupropion, a selective norepinephrine reuptake inhibitor (and likely releasing agent) and nicotinic acetylcholine receptor (nAChR) antagonist that lacks significant dopaminergic actions, and which, with oral bupropion treatment, can reach area under the curve (AUC) plasma concentrations that are as much as 16–20 times greater than those of bupropion itself.[13] Hence, the effects of bupropion cannot be understood unless its metabolism is also considered.[13][15][78]
Dopaminergic activity
The occupancy of dopamine transporter (DAT) sites by bupropion and its metabolites in the human brain as measured by positron emission tomography was 26% according to GlaxoSmithKline researchers and 14% in an independent study.[13][79][80] Despite this weak DAT occupancy however, a subsequent study looked at the actual extracellular concentrations of dopamine in the human brain after an acute oral treatment of bupropion and failed to observe any increase, concluding that the weak DAT occupancy was not sufficient to increase dopamine levels.[13][14] In contrast, the same study also looked at dopamine levels in the rat brain after administration of bupropion via intraperitoneal injection and did see an increase, which could have been related to species differences.[13] However, an alternative explanation is that the difference had to do with the different routes of administration employed (i.e., oral vs. i.p.) and the associated differences in pharmacokinetics and metabolism, namely, the bypassing of first-past metabolism with the latter route, that resulted.[13] Although oral bupropion at clinical doses does not appear to have a significant potential for abuse, there are many isolated case reports of bupropion abuse and "cocaine-like" effects in humans who ingested the drug via a non-oral route (e.g., injection, insufflation, etc.).[81] Notably, awareness of the abuse potential of bupropion via non-conventional routes appears to be especially prominent in correctional facilities.[82]
Antinicotinic and other activities
Bupropion is also known to act as a non-competitive antagonist of the α3β2, α3β4, α4β2, and, very weakly, α7 nACh receptors,[15][83] and these actions appear to be importantly involved in its beneficial properties not only in smoking cessation, but in depression as well.[13][15][77][84] The metabolites of bupropion also act as non-competitive antagonists of these nACh receptors, and hydroxybupropion is even more potent in comparison.[13][85][86][87][88] At therapeutically-relevant concentrations bupropion and hydroxybupropion act as negative allosteric modulators of the serotonin 5-HT3A receptor.[89] Pharmacological data on bupropion and its metabolites are shown in the table. Bupropion is known to weakly inhibit the α1 adrenergic receptor, with a 14% potency of its dopamine uptake inhibition, and the H1 receptor, with a 9% potency.[70]
Pharmacokinetics
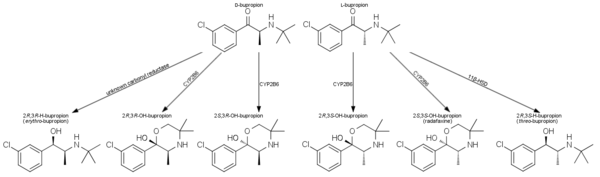
Bupropion is metabolized in the liver by the cytochrome P450 isoenzyme CYP2B6.[48] It has several active metabolites: R,R-hydroxybupropion, S,S-hydroxybupropion, threo-hydrobupropion and erythro-hydrobupropion, which are further metabolized to inactive metabolites and eliminated through excretion into the urine. Both bupropion and its primary metabolite hydroxybupropion act in the liver as potent inhibitors of the enzyme CYP2D6, which metabolizes not only bupropion itself but also a variety of other drugs and biologically active substances.[30] This mechanism creates the potential for a variety of drug interactions.
The biological activity of bupropion can be attributed to a significant degree to its active metabolites, in particular to S,S-hydroxybupropion. GlaxoSmithKline developed this metabolite as a separate drug called radafaxine,[90] but discontinued development in 2006 due to "an unfavourable risk/benefit assessment".[91]
Bupropion is metabolized to hydroxybupropion by CYP2B6, an isozyme of the cytochrome P450 system. Alcohol causes an increase of CYP2B6 in the liver, and persons with a history of alcohol use metabolize bupropion faster. Bupropion is metabolized to threo-hydrobupropion via cortisone reductase.[92] The metabolic pathway responsible for the creation of erythro-hydrobupropion remains elusive.
The metabolism of bupropion is highly variable: the effective doses of bupropion received by persons who ingest the same amount of the drug may differ by as much as 5.5 times (with a half-life of 12–30 hours), while the effective doses of hydroxybupropion may differ by as much as 7.5 times (with a half-life of 15–25 hours).[3][5][93] Based on this, some researchers have advocated monitoring of the blood level of bupropion and hydroxybupropion.[94] The half-lives of erythrohydrobupropion and threohydrobupropion are roughly 23–43 hours and 24–50 hours respectively.[1][3]
There have been reported cases of false-positive urine amphetamine tests in persons taking bupropion.[95][96][97]
In 2016, three new major metabolites of bupropion, all formed exclusively by CYP2C19, were identified.[98] These include 4'-OH-bupropion, erythro-4'-OH-hydrobupropion and threo-4'-OH-hydrobupropion, and represent 24% of a dose of bupropion excreted in urine.[98] For comparison, bupropion and its three previously known primary metabolites, hydroxybupropion, threohydrobupropion, and erythrohydrobupropion represent 23% of a dose of bupropion excreted in urine.[98]
Chemistry
Synthesis
Bupropion is a substituted cathinone. It is synthesized in two chemical steps starting from 3'-chloro-propiophenone. The alpha position adjacent to the ketone is first brominated followed by nucleophilic displacement of the resulting alpha-bromoketone with t-butylamine and treated with hydrochloric acid to give bupropion as the hydrochloride salt in 75–85% overall yield.[99][100]
This diagram shows the synthesis of bupropion via 3'-chloro-propiophenone.
|
History

Bupropion was invented by Nariman Mehta of Burroughs Wellcome (now GlaxoSmithKline) in 1969, and the US patent for it was granted in 1974.[99] It was approved by the United States Food and Drug Administration (FDA) as an antidepressant on 30 December 1985, and marketed under the name Wellbutrin.[101] However, a significant incidence of epileptic seizures at the originally recommended dosage caused the withdrawal of the drug in 1986. Subsequently, the risk of seizures was found to be highly dose-dependent, and bupropion was re-introduced to the market in 1989 with a lower maximum recommended daily dose.[102]
In 1996, the FDA approved a sustained-release formulation of bupropion called Wellbutrin SR, intended to be taken twice a day (as compared with three times a day for immediate-release Wellbutrin).[103] In 2003, the FDA approved another sustained-release formulation called Wellbutrin XL, intended for once-daily dosing. Wellbutrin SR and XL are available in generic form in the United States and Canada. In Canada, generic XR bupropion is distributed by Mylan. In 1997, bupropion was approved by the FDA for use as a smoking cessation aid under the name Zyban.[103] In 2006, Wellbutrin XL was similarly approved as a treatment for seasonal affective disorder.[104]
In France, marketing authorization was granted for Zyban on 3 August 2001, with a maximum daily dose of 300 mg;[105] only sustained-release bupropion is available, and only as a smoking cessation aid. Bupropion was granted a licence for use in adults with major depression in the Netherlands in early 2007, with GlaxoSmithKline expecting subsequent approval in other European countries.[106]
On 11 October 2007, two providers of consumer information on nutritional products and supplements, ConsumerLab.com and The People's Pharmacy, released the results of comparative tests of different brands of bupropion.[107] The People's Pharmacy received multiple reports of increased side effects and decreased efficacy of generic bupropion, which prompted it to ask ConsumerLab.com to test the products in question. The tests showed that "one of a few generic versions of Wellbutrin XL 300 mg, sold as Budeprion XL 300 mg, didn't perform the same as the brand-name pill in the lab."[108] The FDA investigated these complaints and concluded that Budeprion XL is equivalent to Wellbutrin XL in regard to bioavailability of bupropion and its main active metabolite hydroxybupropion. The FDA also said that coincidental natural mood variation is the most likely explanation for the apparent worsening of depression after the switch from Wellbutrin XL to Budeprion XL.[109] On 3 October 2012, however, the FDA reversed this opinion, announcing that "Budeprion XL 300 mg fails to demonstrate therapeutic equivalence to Wellbutrin XL 300 mg."[110] The FDA did not test the bioequivalence of any of the other generic versions of Wellbutrin XL 300 mg, but requested that the four manufacturers submit data on this question to the FDA by March 2013.[110] As of October 2013 the FDA has made determinations on the formulations from some manufacturers not being bioequivalent.[111]
In April 2008, the FDA approved a formulation of bupropion as a hydrobromide salt instead of a hydrochloride salt, to be sold under the name Aplenzin by Sanofi-Aventis.[112]
In 2012, the U.S. Justice Department announced that GlaxoSmithKline had agreed to plead guilty and pay a $3-billion fine, in part for promoting the unapproved use of Wellbutrin for weight loss and sexual dysfunction.[113]
Society and culture
Names
Bupropion is the International Nonproprietary Name (INN) and British Approved Name (BAN) while bupropion hydrochloride is the United States Adopted Name (USAN). Amfebutamone was the former INN.
Recreational use
According to the US government classification of psychiatric medications, bupropion is "non-abusable".[114] However, in animal studies, squirrel monkeys and rats could be induced to self-administer bupropion intravenously, which is often taken as a sign of addiction potential.[30] There have been a number of anecdotal and case-study reports of bupropion abuse, but the bulk of evidence indicates that the subjective effects of bupropion via the oral route are markedly different from those of addictive stimulants such as cocaine or amphetamine.[115] That said, bupropion, via non-conventional routes of administration (e.g., injection, insufflation), is reported to be abused in the United States and Canada, notably in prisons.[116][117][118]
Brands
It is sold under many trade names worldwide and in combinations with naltrexone.[119]
| Brand name listings |
|---|
|
It is sold under many trade names worldwide including Aplenzin, Budeprion SR, Bup, Bupredol, Buproban, Bupropion GSK, BuPROPion HCL SR Watson, Bupropion Hydrochloride Anchen, Bupropion Hydrochloride Apotex, BuPROPion Hydrochloride Cadista, Bupropion Hydrochloride Mylan, Bupropion Hydrochloride Sandoz, buPROPion Hydrochloride SR actavis, Bupropion Hydrochloride Sun Pharma, buPROPion Hydrochloride Torrent Pharma, Bupropion Hydrochloride Wockhardt, buPROPion Hydrochloride XL actavis, BuPROPion Hydrochloride XL Watson, Bupropion SR Sanis Health, Bupropionhydrochlorid HEXAL, Bupropionhydrochloride GSK, Buprotrin, Butrin, Buxon, Carmubine, Depnox-SR, Elontril, Elontril XL, Forfivo XL, Funnix, Global buPROPion HCL, Le Fu Ting, Odranal, PMS-Bupropion SR, Prewell, Quomem, ratio-Bupropion SR, Sandoz Bupropion SR, Voxra, Wellbutrin, Wellbutrin Retard, Wellbutrin SR, Wellbutrin XL, Wellbutrin XR, Yue Ting, Zetron, Zyban, Zyban LP, Zybex SR, ZyGenerics Bupropion Hydrochloride XL, and Zyntabac.[119] It is sold as a combination drug with naltrexone as Contrave.[119] |
References
- 1 2 3 4 5 6 7 "Zyban 150 mg prolonged release film-coated tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. GlaxoSmithKline UK. 1 August 2013. Retrieved 22 October 2013.
- 1 2 3 "Prexaton Bupropion hydrochloride PRODUCT INFORMATION". TGA eBusiness Services. Ascent Pharma Pty Ltd. 2 October 2012. Retrieved 22 October 2013.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 FDA Buproprion Label Last revised December 16, 2014 as described on the FDA Label Website. See that site for updates. Page accessed April 8, 2016
- ↑ Zhu, A. Z. X.; Zhou, Q.; Cox, L. S.; Ahluwalia, J. S.; Benowitz, N. L.; Tyndale, R. F. (3 September 2014). "Gene Variants in CYP2C19 Are Associated with Altered In Vivo Bupropion Pharmacokinetics but Not Bupropion-Assisted Smoking Cessation Outcomes". Drug Metabolism and Disposition. 42 (11): 1971–1977. PMC 4201132
 . PMID 25187485. doi:10.1124/dmd.114.060285.
. PMID 25187485. doi:10.1124/dmd.114.060285. - 1 2 Brunton, L, Chabner, B, Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- ↑ "Bupropion". PubChem.
- ↑ Morton, Ian, Morton, I.K., Hall, Judith M. (1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 57–. ISBN 978-0-7514-0499-9.
- ↑ Dictionary of Organic Compounds. CRC Press. pp. 104–. ISBN 978-0-412-54090-5.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 38–. ISBN 978-3-88763-075-1.
- 1 2 "Bupropion (By mouth)". PubMed Health. Bethesda, USA: National Institute of Health. 1 June 2014. Retrieved 18 July 2014.
- 1 2 Brayfield, A, ed. (22 October 2013). "Bupropion". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 18 July 2014.
- 1 2 3 4 5 Fava M, Rush AJ, Thase ME, et al. (2005). "15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL". Prim Care Companion J Clin Psychiatry. 7 (3): 106–13. PMC 1163271
 . PMID 16027765. doi:10.4088/pcc.v07n0305.
. PMID 16027765. doi:10.4088/pcc.v07n0305. - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Dwoskin, Linda P. (29 January 2014). Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Elsevier Science. pp. 177–216. ISBN 978-0-12-420177-4.
- 1 2 Tasman, Allan, Kay, Jerald, Lieberman, Jeffrey A., First, Michael B., Maj, Mario (11 October 2011). Psychiatry. John Wiley & Sons. ISBN 978-1-119-96540-4.
- 1 2 3 4 5 6 7 Lemke, Thomas L., Williams, David A. (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 611–613. ISBN 978-1-60913-345-0.
- ↑ The INN originally assigned in 1974 by the World Health Organization was "amfebutamone". In 2000, the INN was reassigned as bupropion. See World Health Organization (2000). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 83" (PDF). WHO Drug Information. 14 (2). Archived from the original (PDF) on 1 February 2010. Retrieved 22 June 2009.
- ↑ Moreira R (October 2011). "The efficacy and tolerability of bupropion in the treatment of major depressive disorder". Clin Drug Investig. 31 Suppl 1: 5–17. PMID 22015858. doi:10.2165/1159616-S0-000000000-00000.
- 1 2 3 Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C (February 2009). "Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis" (PDF). Lancet. 373 (9665): 746–758. PMID 19185342. doi:10.1016/S0140-6736(09)60046-5.
- ↑ Clayton AH (2003). "Antidepressant-Associated Sexual Dysfunction: A Potentially Avoidable Therapeutic Challenge". Primary Psychiatry. 10 (1): 55–61.
- ↑ Dhillon S, Yang LP, Curran MP (2008). "Bupropion: a review of its use in the management of major depressive disorder". Drugs. 68 (5): 653–89. PMID 18370448. doi:10.2165/00003495-200868050-00011.
- ↑ Baldwin DS, Papakostas GI (2006). "Symptoms of fatigue and sleepiness in major depressive disorder". J Clin Psychiatry. 67 Suppl 6 (Suppl 6): 9–15. PMID 16848671.
- ↑ Papakostas GI, Stahl SM, Krishen A, Seifert CA, Tucker VL, Goodale EP, Fava M (August 2008). "Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies". J Clin Psychiatry. 69 (8): 1287–92. PMID 18605812. doi:10.4088/JCP.v69n0812.
- 1 2 Zisook S, Rush AJ, Haight BR, Clines DC, Rockett CB (February 2006). "Use of bupropion in combination with serotonin reuptake inhibitors". Biol. Psychiatry. 59 (3): 203–10. PMID 16165100. doi:10.1016/j.biopsych.2005.06.027.
- ↑ "First drug for seasonal depression". FDA Consum. 40 (5): 7. 2006. PMID 17328102.
- 1 2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 Joint Formulary Committee (2015). British National Formulary (BNF) (69 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-156-2.
- ↑ Wilkes, S (2008). "The use of bupropion SR in cigarette smoking cessation.". International journal of chronic obstructive pulmonary disease. 3 (1): 45–53. PMC 2528204
 . PMID 18488428. doi:10.2147/copd.s1121.
. PMID 18488428. doi:10.2147/copd.s1121. - ↑ Hughes, JR; Stead, LF; Hartmann-Boyce, J; Cahill, K; Lancaster, T (8 January 2014). "Antidepressants for smoking cessation.". The Cochrane database of systematic reviews. 1: CD000031. PMID 24402784. doi:10.1002/14651858.CD000031.pub4.
- 1 2 3 Wu P, Wilson K, Dimoulas P, Mills EJ (2006). "Effectiveness of smoking cessation therapies: a systematic review and meta-analysis". BMC Public Health. 6: 300. PMC 1764891
 . PMID 17156479. doi:10.1186/1471-2458-6-300.
. PMID 17156479. doi:10.1186/1471-2458-6-300. - 1 2 3 4 Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT (2006). "Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent". CNS Drug Rev. 12 (3–4): 178–207. PMID 17227286. doi:10.1111/j.1527-3458.2006.00178.x.
- ↑ Kornfield R, Watson S, Higashi A, Dusetzina S, Conti R, Garfield R, Dorsey ER, Huskamp HA, Alexander GC (April 2013). "Impact of FDA Advisories on Pharmacologic Treatment of Attention Deficit Hyperactivity Disorder". Psychiatric Services. 64 (4): 339–46. PMC 4023684
 . PMID 23318985. doi:10.1176/appi.ps.201200147.
. PMID 23318985. doi:10.1176/appi.ps.201200147. - 1 2 Cantwell DP (1998). "ADHD through the life span: the role of bupropion in treatment". J Clin Psychiatry. 59 Suppl 4: 92–4. PMID 9554326.
- ↑ Pliszka S (July 2007). "Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder". J Am Acad Child Adolesc Psychiatry. 46 (7): 894–921. PMID 17581453. doi:10.1097/chi.0b013e318054e724.
- ↑ Pliszka SR, Crismon ML, Hughes CW, Corners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M (June 2006). "The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder". J Am Acad Child Adolesc Psychiatry. 45 (6): 642–57. PMID 16721314. doi:10.1097/01.chi.0000215326.51175.eb.
- ↑ Serretti A, Chiesa A (June 2009). "Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis". J Clin Psychopharmacol. 29 (3): 259–66. PMID 19440080. doi:10.1097/JCP.0b013e3181a5233f.
- ↑ Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004). "A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor". Prim Care Companion J Clin Psychiatry. 6 (4): 159–166. PMC 514842
 . PMID 15361919. doi:10.4088/PCC.v06n0403.
. PMID 15361919. doi:10.4088/PCC.v06n0403. - ↑ Foley KF, DeSanty KP, Kast RE (September 2006). "Bupropion: pharmacology and therapeutic applications". Expert Rev Neurother. 6 (9): 1249–65. PMID 17009913. doi:10.1586/14737175.6.9.1249.
- 1 2 Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC (April 2005). "Meta-analysis: pharmacologic treatment of obesity". Ann. Intern. Med. 142 (7): 532–46. PMID 15809465. doi:10.7326/0003-4819-142-7-200504050-00012.
- 1 2 Ryan DH, Bray GA (June 2013). "Pharmacologic treatment options for obesity: what is old is new again.". Current hypertension reports. 15 (3): 182–9. PMID 23625271. doi:10.1007/s11906-013-0343-6.
- ↑ "Contrave label" (PDF). United States Food and Drug Administration. 10 September 2014. p. 1. Retrieved 6 April 2015.
- ↑ Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM (September 2004). "Antidepressants for bipolar depression: a systematic review of randomized, controlled trials". Am J Psychiatry. 161 (9): 1537–47. PMID 15337640. doi:10.1176/appi.ajp.161.9.1537.
- ↑ Yatham LN, Kennedy SH, O'Donovan C, Parikh SV, MacQueen G, McIntyre RS, Sharma V, Beaulieu S (December 2006). "Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007". Bipolar Disord. 8 (6): 721–39. PMID 17156158. doi:10.1111/j.1399-5618.2006.00432.x.
- ↑ Kampman KM (June 2008). "The search for medications to treat stimulant dependence". Addict Sci Clin Pract. 4 (2): 28–35. PMC 2797110
 . PMID 18497715. doi:10.1151/ascp084228.
. PMID 18497715. doi:10.1151/ascp084228. - ↑ Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ (2006). "Antidepressants and inflammatory bowel disease: a systematic review". Clin Pract Epidemiol Ment Health. 2: 24. PMC 1599716
 . PMID 16984660. doi:10.1186/1745-0179-2-24.
. PMID 16984660. doi:10.1186/1745-0179-2-24. - ↑ "FDA clears Cymbalta to treat chronic musculoskeletal pain". FDA Press Announcements. Food and Drug Administration. 4 November 2010. Retrieved 19 August 2013.
The U.S. Food and Drug Administration ... approved Cymbalta (duloxetine hydrochloride) to treat chronic musculoskeletal pain, including discomfort from osteoarthritis and chronic lower back pain.
- ↑ Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW (2008). Urquhart DM, ed. "Antidepressants for non-specific low back pain". Cochrane Database Syst Rev (1): CD001703. PMID 18253994. doi:10.1002/14651858.CD001703.pub3.
- ↑ Shah TH, Moradimehr A (August 2010). "Bupropion for the treatment of neuropathic pain". Am J Hosp Palliat Care. 27 (5): 333–6. PMID 20185402. doi:10.1177/1049909110361229.
- 1 2 3 Hales E, Yudofsky JA, eds. (2003). The American Psychiatric Press Textbook of Psychiatry. Washington, DC: American Psychiatric Publishing, Inc.
- ↑ Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R (2002). "Effects of psychotropic drugs on seizure threshold". Drug Saf. 25 (2): 91–110. PMID 11888352. doi:10.2165/00002018-200225020-00004.
- 1 2 Alper K, Schwartz KA, Kolts RL, Khan A (August 2007). "Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports". Biol. Psychiatry. 62 (4): 345–54. PMID 17223086. doi:10.1016/j.biopsych.2006.09.023.
- ↑ Taylor D (December 2008). "Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety". Acta Psychiatr Scand. 118 (6): 434–42. PMID 18785947. doi:10.1111/j.1600-0447.2008.01260.x.
- ↑ "Zyban (bupropion hydrochloride) – safety update". Medicines and Healthcare products Regulatory Agency. 24 July 2002. Archived from the original on 28 September 2007. Retrieved 7 October 2006.
- ↑ Hubbard R, Lewis S, West J, Smith C, Godfrey C, Smeeth L, Farrington P, Britton J (October 2005). "Bupropion and the risk of sudden death: a self-controlled case-series analysis using The Health Improvement Network". Thorax. 60 (10): 848–50. PMC 1747199
 . PMID 16055620. doi:10.1136/thx.2005.041798.
. PMID 16055620. doi:10.1136/thx.2005.041798. - 1 2 Levenson M, Holland C. "Antidepressants and suicidality in adults: statistical evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". U.S. Food and Drug Administration. Retrieved 13 May 2007.
- ↑ Hughes, JR; Stead, LF; Hartmann-Boyce, J; Cahill, K; Lancaster, T (8 January 2014). "Antidepressants for smoking cessation.". The Cochrane database of systematic reviews. 1: CD000031. PMID 24402784. doi:10.1002/14651858.CD000031.pub4.
- ↑ Commissioner, Office of the. "Safety Alerts for Human Medical Products - Chantix (varenicline) and Zyban (bupropion): Drug Safety Communication - Mental Health Side Effects Revised". www.fda.gov. Retrieved 20 December 2016.
- ↑ "Public Health Advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban". U.S. Food and Drug Administration (FDA). 1 July 2009. Archived from the original on 19 October 2010. Retrieved 3 July 2009.
- ↑ "The smoking cessation aids varenicline (marketed as Chantix) and bupropion (marketed as Zyban and generics) suicidal ideation and behavior" (PDF). Drug Safety Newsletter. U.S. Food and Drug Administration (FDA). 2 (1): 1–4. 2009.
- 1 2 3 Kumar S, Kodela S, Detweiler JG, Kim KY, Detweiler MB (November–December 2011). "Bupropion-induced psychosis: folklore or fact? A Systematic Review of the Literature". Gen Hosp Psychiatry. 33 (12): 612–7. PMID 21872337. doi:10.1016/j.genhosppsych.2011.07.001.
- ↑ Javelot T, Javelot H, Baratta A, Weiner L, Messaoudi M, Lemoine P (December 2010). "Acute psychotic disorders related to bupropion: review of the literature". Encephale. 36 (6): 461–71. PMID 21130229. doi:10.1016/j.encep.2010.01.005.
- ↑ Nemeroff CB, Schatzberg AF (2006). Essentials of clinical psychopharmacology. Washington, D.C: American Psychiatric Publishing. p. 146. ISBN 1-58562-243-5.
- ↑ Johnston JA (1999). "Discontinuation of Therapy With Bupropion SR". Prim Care Companion J Clin Psychiatry. 1 (5): 165. PMC 181084
 . PMID 15014680.
. PMID 15014680. - ↑ Berigan TR (April 2002). "Bupropion-associated withdrawal symptoms revisited: a case report". Prim Care Companion J Clin Psychiatry. 4 (2): 78. PMC 181231
 . PMID 15014751. doi:10.4088/PCC.v04n0208a.
. PMID 15014751. doi:10.4088/PCC.v04n0208a. - ↑ Taylor D, Carol P, Shitij K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97969-3.
- ↑ White N, Litovitz T, Clancy C (December 2008). "Suicidal antidepressant overdoses: a comparative analysis by antidepressant type". Journal of Medical Toxicology. 4 (4): 238–250. PMC 3550116
 . PMID 19031375. doi:10.1007/BF03161207.
. PMID 19031375. doi:10.1007/BF03161207. - ↑ Beuhler MC, Spiller HA, Sasser HC (March 2010). "The outcome of unintentional pediatric bupropion ingestions: a NPDS database review". J Med Toxicol. 6 (1): 4–8. PMC 3550434
 . PMID 20213217. doi:10.1007/s13181-010-0027-4.
. PMID 20213217. doi:10.1007/s13181-010-0027-4. - 1 2 3 Jefferson JW, Pradko JF, Muir KT (November 2005). "Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations". Clin Ther. 27 (11): 1685–95. PMID 16368442. doi:10.1016/j.clinthera.2005.11.011.
- ↑ Fidel Vila-Rodriguez, Fidel; Dobek, Christine E; Blumberger, Daniel M; Downar, Jonathan; Daskalakis, Zafiris J (2015). "Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion". Neuropsychiatric Disease and Treatment: 2975. ISSN 1178-2021. doi:10.2147/NDT.S91126.
- ↑ Feinberg SS (2004). "Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication". J Clin Psychiatry. 65 (11): 1520–4. PMID 15554766. doi:10.4088/jcp.v65n1113.
- 1 2 Horst WD, Preskorn SH (December 1998). "Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion". J Affect Disord. 51 (3): 237–54. PMID 10333980. doi:10.1016/S0165-0327(98)00222-5.
- ↑ Johnston AJ, Ascher J, Leadbetter R, Schmith VD, Patel DK, Durcan M, Bentley B (2002). "Pharmacokinetic optimisation of sustained-release bupropion for smoking cessation". Drugs. 62 Suppl 2: 11–24. PMID 12109932. doi:10.2165/00003495-200262002-00002.
- ↑ Xu H, Loboz KK, Gross AS, McLachlan AJ (March 2007). "Stereoselective analysis of hydroxybupropion and application to drug interaction studies". Chirality. 19 (3): 163–70. PMID 17167747. doi:10.1002/chir.20356.
- ↑ Bondarev ML, Bondareva TS, Young R, Glennon RA (August 2003). "Behavioral and biochemical investigations of bupropion metabolites". Eur. J. Pharmacol. 474 (1): 85–93. PMID 12909199. doi:10.1016/S0014-2999(03)02010-7.
- ↑ Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR (September 2004). "Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors". Mol. Pharmacol. 66 (3): 675–82. PMID 15322260. doi:10.1124/mol.104.001313.
- ↑ Arias HR, Santamaría A, Ali SF (2009). "Pharmacological and neurotoxicological actions mediated by bupropion and diethylpropion". Int. Rev. Neurobiol. 88: 223–55. PMID 19897080. doi:10.1016/S0074-7742(09)88009-4.
- ↑ Labbate, Lawrence A., Fava, Maurizio, Rosenbaum, Jerrold F., Arana, George W. (28 March 2012). Handbook of Psychiatric Drug Therapy. Lippincott Williams & Wilkins. pp. 64–. ISBN 978-1-4511-5307-1.
- 1 2 Warner C, Shoaib M (September 2005). "How does bupropion work as a smoking cessation aid?". Addict Biol. 10 (3): 219–31. PMID 16109583. doi:10.1080/13556210500222670.
- ↑ Ascher JA, Cole JO, Colin JN et al. (September 1995). "Bupropion: a review of its mechanism of antidepressant activity". J Clin Psychiatry. 56 (9): 395–401. PMID 7665537.
- ↑ Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B (October 2003). "In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography". Biol. Psychiatry. 54 (8): 800–5. PMID 14550679. doi:10.1016/S0006-3223(02)01834-6.
- ↑ Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S (August 2002). "Bupropion occupancy of the dopamine transporter is low during clinical treatment". Psychopharmacology (Berl.). 163 (1): 102–5. PMID 12185406. doi:10.1007/s00213-002-1166-3.
- ↑ Oppek K, Koller G, Zwergal A, Pogarell O (June 2014). "Intravenous Administration and Abuse of Bupropion: A Case Report and a Review of the Literature". J Addict Med. 8 (4): 290–3. PMID 24950138. doi:10.1097/ADM.0000000000000044.
- ↑ Hilliard WT, Barloon L, Farley P, Penn JV, Koranek A (July 2013). "Bupropion diversion and misuse in the correctional facility". J Correct Health Care. 19 (3): 211–7. PMID 23788587. doi:10.1177/1078345813486448.
- ↑ Carroll FI, Blough BE, Mascarella SW, Navarro HA, Lukas RJ, Damaj MI (2014). "Bupropion and bupropion analogs as treatments for CNS disorders". Adv. Pharmacol. 69: 177–216. PMID 24484978. doi:10.1016/B978-0-12-420118-7.00005-6.
- ↑ Arias HR (2009). "Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions?". Int. J. Biochem. Cell Biol. 41 (11): 2098–108. PMID 19497387. doi:10.1016/j.biocel.2009.05.015.
- ↑ Damaj MI, Carroll FI, Eaton JB, et al. (September 2004). "Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors". Mol. Pharmacol. 66 (3): 675–82. PMID 15322260. doi:10.1124/mol.104.001313.
- ↑ Zhu AZ, Cox LS, Nollen N, et al. (December 2012). "CYP2B6 and bupropion's smoking-cessation pharmacology: the role of hydroxybupropion". Clin. Pharmacol. Ther. 92 (6): 771–7. PMC 3729209
 . PMID 23149928. doi:10.1038/clpt.2012.186.
. PMID 23149928. doi:10.1038/clpt.2012.186. - ↑ Foxhall, Lewis E.; Rodriguez, Maria Alma (2014). Advances in Cancer Survivorship Management. Springer. pp. 265–. ISBN 978-1-4939-0986-5.
- ↑ Johnson, Bankole A. (10 October 2010). Addiction Medicine: Science and Practice. Springer Science & Business Media. pp. 433–. ISBN 978-1-4419-0338-9.
- ↑ Pandhare A, Pappu AS, Wilms H, Blanton MP, Jansen M (2016). "The antidepressant bupropion is a negative allosteric modulator of serotonin type 3A receptors". Neuropharmacology. 113 (Pt A): 89–99. PMID 27671323. doi:10.1016/j.neuropharm.2016.09.021.
- ↑ "GlaxoSmithKline (GSK) Reviews Novel Therapeutics For CNS Disorders And Confirms Strong Pipeline Momentum" (Press release). PRNewswire. 23 November 2004. Retrieved 18 August 2007.
- ↑ GlaxoSmithKline (26 July 2006) "Pipeline Update" (PDF). Archived from the original (PDF) on 27 September 2007. (136 KB). Press release. Retrieved on 18 August 2007.
- ↑ Meyer A, Vuorinen A, Zielinska AE, Strajhar P, Lavery GG, Schuster D, Odermatt A (September 2013). "Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1.". Drug metabolism and disposition: the biological fate of chemicals. 41 (9): 1671–8. PMC 3876805
 . PMID 23804523. doi:10.1124/dmd.113.052936.
. PMID 23804523. doi:10.1124/dmd.113.052936. - ↑ Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH (April 2004). "Pharmacogenetic determinants of inter-individual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes". Pharmacogenetics. 14 (4): 225–38. PMID 15083067. doi:10.1097/00008571-200404000-00002.
- ↑ Preskorn SH (1991). "Should bupropion dosage be adjusted based upon therapeutic drug monitoring?". Psychopharmacol Bull. 27 (4): 637–43. PMID 1813908.
- ↑ Weintraub D, Linder MW (2000). "Amphetamine positive toxicology screen secondary to bupropion". Depress Anxiety. 12 (1): 53–4. PMID 10999247. doi:10.1002/1520-6394(2000)12:1<53::AID-DA8>3.0.CO;2-4.
- ↑ Nixon AL, Long WH, Puopolo PR, Flood JG (June 1995). "Bupropion metabolites produce false-positive urine amphetamine results". Clin. Chem. 41 (6 Pt 1): 955–6. PMID 7768026.
- ↑ Casey, E.R.; Scott, M.G.; Tang, S.; Mullins, M.E. (Jun 2011). "Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay". J Med Toxicol. 7: 105–8. PMC 3724447
 . PMID 21191682. doi:10.1007/s13181-010-0131-5.
. PMID 21191682. doi:10.1007/s13181-010-0131-5. - 1 2 3 Sager JE, Choiniere JR, Chang J, Stephenson-Famy A, Nelson WL, Isoherranen N (2016). "Identification and Structural Characterization of Three New Metabolites of Bupropion in Humans". ACS Med Chem Lett. 7 (8): 791–6. PMID 27660681. doi:10.1021/acsmedchemlett.6b00189.
- 1 2 Mehta NB (25 June 1974). "United States Patent 3,819,706: Meta-chloro substituted α-butylamino-propiophenones". USPTO. Retrieved 2 June 2008.
- ↑ Perrine DM, Ross JT, Nervi SJ, Zimmerman RH (2000). "A Short, One-Pot Synthesis of Bupropion (Zyban, Wellbutrin)". Journal of Chemical Education. 77 (11): 1479. Bibcode:2000JChEd..77.1479P. doi:10.1021/ed077p1479.
- ↑ "Wellbutrin entry in the Orange Book". U.S. Food and Drug Administration Center for Drug Evaluation and Research. Retrieved 18 August 2007.
- ↑ "Bupropion (Wellbutrin)". eMedExpert.com. 31 March 2008. Retrieved 20 August 2013.
- 1 2 Whitten L (April 2006). "Bupropion helps people with schizophrenia quit smoking". National Institute on Drug Abuse Research Findings. 20 (5). Archived from the original on 5 August 2007. Retrieved 27 May 2013.
- ↑ "Seasonal affective disorder drug Wellbutrin XL wins approval". CNN. 14 June 2006. Archived from the original on 30 June 2007. Retrieved 19 August 2007.
- ↑ "Zyban : sevrage tabagique et sécurité d'emploi" [Zyban: smoking cessation and job security] (Press release) (in French). Agence française de sécurité sanitaire des produits de santé. 18 January 2002. Retrieved 25 January 2011.
- ↑ GlaxoSmithKline (16 January 2007). "GlaxoSmithKline receives first European approval for Wellbutrin XR" (Press release). GlaxoSmithKline. Archived from the original on 27 December 2010. Retrieved 25 January 2011.
- ↑ "Generic drug equality questioned". Retrieved 13 October 2007.
- ↑ Stenson, Jacqueline (12 October 2007). "Report questions generic antidepressant". msnbc.com. Retrieved 13 October 2007.
- ↑ "Review of therapeutic equivalence: generic bupropion XL 300 mg and Wellbutrin XL 300 mg". Archived from the original on 6 June 2011. Retrieved 19 April 2008.
- 1 2 "Budeprion XL 300 mg not therapeutically equivalent to Wellbutrin XL 300 mg" (Press release). FDA. 3 October 2012. Retrieved 23 March 2013.
- ↑ "FDA Update". FDA. October 2013. Retrieved 15 June 2015.
- ↑ Waknine, Yael (8 May 2008). "FDA Approvals: Advair, Relistor, Aplenzin". Medscape. Retrieved 9 May 2008.
- ↑ Thomas K, Schmidt MS (2 July 2012). "Glaxo agrees to pay $3 billion in fraud settlement". The New York Times.
- ↑ "Abuse potential of common psychiatric medications". Substance abuse treatment for persons with HIV/AIDS. Treatment Improvement Protocol. Rockville: Substance Abuse and Mental Health Services Administration. pp. 83–4.
- ↑ Lile JA, Nader MA (2003). "The abuse liability and therapeutic potential of drugs evaluated for cocaine addiction as predicted by animal models". Current Neuropharmacology. 1: 21–46. doi:10.2174/1570159033360566.
- ↑ Antidepressant Wellbutrin becomes 'poor man's cocaine' on Toronto streets Global News 18 September 2013.
- ↑ Philipps, DeAnne (February 2012). "Wellbutrin®: Misuse and Abuse by Incarcerated Individuals". Journal of Addictions Nursing. 23 (1): 65–69. PMID 22468662. doi:10.3109/10884602.2011.647838.
- ↑ Baribeau, Danielle; Araki, Keyghobad Farid (May–June 2013). "Intravenous Bupropion: A Previously Undocumented Method of Abuse of a Commonly Prescribed Antidepressant Agent". Journal of Addiction Medicine. 7 (3): 216–217. PMID 23519045. doi:10.1097/ADM.0b013e3182824863.
- 1 2 3 "Bupropion International Brands". Drugs.com. Retrieved 1 June 2017.
External links
| Wikimedia Commons has media related to Bupropion. |
-bupropion_ball-and-stick_xtal_2009.png)