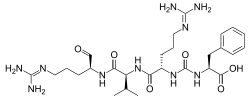
Antipain
 | |
| Names | |
|---|---|
| IUPAC name
N2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-N5-(diaminomethylidene)-L-ornithyl-N-{(2S)-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl}-L-valinamide | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.048.738 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C27H44N10O6 | |
| Molar mass | 604.71 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Antipain is an oligopeptide isolated from actinomycetes and used in biochemical research as a protease inhibitor, reported in 1972 as the first natural peptide containing a ureylene group.[1] Specifically, it is an inhibitor of trypsin and papain.[2]
It has been crystallised in complex with carboxypeptidase from wheat[3] and Leishmania major oligopeptidase B.[4] In both cases the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
References
- ↑ Umezawa, S; Tatsuta, K; Fujimoto, K; Tsuchiya, T; Umezawa, H (1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of antibiotics. 25 (4): 267–70. PMID 5052959. doi:10.7164/antibiotics.25.267.
- ↑ Suda, H; Aoyagi, T; Hamada, M; Takeuchi, T; Umezawa, H (1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of antibiotics. 25 (4): 263–6. PMID 4559651. doi:10.7164/antibiotics.25.263.
- ↑ PDB ENTRY 1bcr Bullock, TL; Breddam, K; Remington, SJ (1996). "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity.". J.Mol.Biol. 255 (5): 714–25. PMID 8636973. doi:10.1006/jmbi.1996.0058.
- ↑ PDB ENTRY 2xe4 McLuskey, K; Paterson, NG; Bland, ND; Isaacs, NW; Mottram, JC (2010). "Crystal Structure of Leishmania Major Oligopeptidase B Gives Insight Into the Enzymatic Properties of a Trypanosomatid Virulence Factor.". J.Biol. Chem. 285 (50): 39249–59. PMC 2998157
 . PMID 20926390. doi:10.1074/jbc.M110.156679.
. PMID 20926390. doi:10.1074/jbc.M110.156679.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.