Ampicillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Principen, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% (oral) |
| Protein binding | 15 to 25% |
| Metabolism | 12 to 50% |
| Biological half-life | approx 1 hour |
| Excretion | 75 to 85% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.645 |
| Chemical and physical data | |
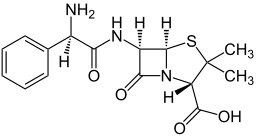
| Formula | C16H19N3O4S |
| Molar mass | 349.41 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously.[2] Like all antibiotics, it is not useful for the treatment of viral infections.
Common side effects include rash, nausea, and diarrhea. It should not be used in people who are allergic to penicillin. Serious side effects may include Clostridium difficile colitis or anaphylaxis. While usable in those with kidney problems, the dose may need to be decreased.[2] Its use during pregnancy and breastfeeding appears to be generally safe.[2][3]
Ampicillin was discovered in 1958 and came into commercial use in 1961.[4][5] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] Its wholesale cost in the developing world is between US$0.13 and 1.20 for a vial of the intravenous solution as of 2014.[7] In the United States, it is available as a generic medication and 10 days of treatment cost about $13.[2]
Medical uses
Ampicillin is used to treat infections by many Gram-positive and Gram-negative bacteria. Ampicillin was the first 'broad spectrum' penicillin with activity against Gram-positive bacteria including Streptococcus pneumoniae, Streptococcus pyogenes, some isolates of Staphylococcus aureus (but not penicillin-resistant or methicillin-resistant strains), and some Enterococcus. Activity against Gram-negative bacteria includes Neisseria meningitidis, some Haemophilus influenzae, and some of the Enterobacteriaceae. Its spectrum of activity is enhanced by co-administration of sulbactam, a drug that inhibits beta lactamase, an enzyme produced by bacteria to inactivate ampicillin and related antibiotics.[8][9] It is sometimes used in combination with other antibiotics that have different mechanisms of action, like vancomycin, linezolid, daptomycin, and tigecycline.[10][11]
Ampicillin can be administered by mouth, an intramuscular injection (shot) or by intravenous infusion.[2]
Indications
- respiratory infections
- sinusitis
- bronchitis
- pharyngitis
- otitis media
- bacterial meningitis[12]
- Salmonella, Shigella, and Listeria bacteria.
- gastrointestinal infections caused by contaminated water or food.
- healthcare-associated infections related to infection from urinary catheter use unresponsive to other medications.[13]
- Haemophilus influenzae, Streptococcus pneumoniae infections.
- Genito-urinary tract infections
- prevent infection (prophylaxis) in those who previously had rheumatic heart disease or are undergoing dental procedures.[12]
Side effects
Ampicillin is comparatively less toxic than other antibiotics. In very rare cases, it causes severe side effects such as angioedema, anaphylaxis, and C. difficile infection. Some develop black 'furry' tongue'. The most serious adverse effects are seizures, serum sickness, anaphylaxis, pseudomembranous colitis. The most common side effects to be expected in ten percent of users are diarrhea and rash. Less common side effects can be nausea, vomiting, itching, blood dyscrasias, and mild allergic reactions. Other conditions may develop up several weeks after treatment.[2][12]
Interactions
Ampicillin reacts with probenecid to decrease renal excretion. Large doses of ampicillin can increase the risk of bleeding with concurrent use of warfarin. Ampicillin use can make oral contraceptives less effective.[2][12]
Mechanisms
Ampicillin is in the penicillin group of beta-lactam antibiotics and is part of the aminopenicillin family. It is roughly equivalent to amoxicillin in terms of activity.[2]
Ampicillin is able to penetrate Gram-positive and some Gram-negative bacteria. It differs from penicillin G, or benzylpenicillin, only by the presence of an amino group. That amino group helps the drug penetrate the outer membrane of Gram-negative bacteria.
Ampicillin acts as an irreversible inhibitor of the enzyme transpeptidase, which is needed by bacteria to make the cell wall.[2] It inhibits the third and final stage of bacterial cell wall synthesis in binary fission, which ultimately leads to cell lysis; therefore, ampicillin is usually bacteriolytic.[2][14]
History
Ampicillin has been used extensively to treat bacterial infections since 1961.[15] Until the introduction of ampicillin by the British company Beecham, penicillin therapies had only been effective against Gram-positive organisms such as staphylococci and streptococci.[14] Ampicillin (originally branded as 'Penbritin') also demonstrated activity against Gram-negative organisms such as H. influenzae, coliforms, and Proteus spp.[15]
Cost
Its wholesale costs is between US$0.13 and 1.20 for a vial of the intravenous solution as of 2014.[7] In the United States, it is available as a generic medication and 10 days of treatment cost about $13.[2]
See also
- Amoxycillin (p-hydroxy metabolite of ampicillin)
- Pivampicillin (special pro-drug of ampicillin)
- Azlocillin and pirbenicillin (urea and amide made from ampicillin)
References
- ↑ Drugs.com International trade names for Ampicillin Retrieved 14 January 2015
- 1 2 3 4 5 6 7 8 9 10 11 "Ampicillin". The American Society of Health-System Pharmacists. Retrieved 1 August 2015.
- ↑ "Ampicillin use while Breastfeeding". March 2015. Retrieved 1 August 2015.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 9783527607495.
- ↑ Ravina, Enrique (2011). The evolution of drug discovery : from traditional medicines to modern drugs (1 ed.). Weinheim: Wiley-VCH. p. 262. ISBN 9783527326693.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- 1 2 "Ampicillin". International Drug Price Indicator Guide. Retrieved 1 August 2015.
- ↑ Hauser AR (2013). Antibiotic Basics for Clinicians, 2nd ed. Lippincott, Williams, and Wikins, pp. 25–28
- ↑ Akova M (January 2008). "Sulbactam-containing beta-lactamase inhibitor combinations". Clin. Microbiol. Infect. 14 Suppl 1: 185–8. PMID 18154545. doi:10.1111/j.1469-0691.2007.01847.x.
- ↑ Suleyman, G; Zervos, MJ (2016). "Safety and efficacy of commonly used antimicrobial agents in the treatment of enterococcal infections: a review.". Expert opinion on drug safety. 15 (2): 153–67. PMID 26629598. doi:10.1517/14740338.2016.1127349.
- ↑ Torok, M. Estee; Moran, Ed; Cooke, Fiona. Oxford Handbook of Infectious Diseases and Microbiology. Oxford University Press. p. 721. ISBN 9780191503108.
- 1 2 3 4 "Ampicillin". Davis. 2017. Retrieved March 22, 2017.
- ↑ Finberg R, Fingeroth J in Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo (eds.) (2012) Harrison's Principles of Internal Medicine, 18th ed., McGraw-Hill, Chapter 132.
- 1 2 Petri WA in Brunton LL, Chabner BA, Knollmann BC (eds.) (2011 ) Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th ed., Chapter 53. McGraw-Hill, New York.
- 1 2 Acred, P; Brown, D. M.; Turner, D. H.; Wilson, M. J. (April 1962). "Pharmacology and chemotherapy of ampicillin--a new broad-spectrum penicillin". Br J Pharmacol Chemother. 18 (2): 356–69. PMC 1482127
 . PMID 13859205. doi:10.1111/j.1476-5381.1962.tb01416.x.
. PMID 13859205. doi:10.1111/j.1476-5381.1962.tb01416.x.
External links
- Ampicillin bound to proteins in the PDB