Amikacin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Amikin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682661 |
| Pregnancy category | |
| Routes of administration | intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 0-11% |
| Biological half-life | 2-3 hours |
| Excretion | kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.048.653 |
| Chemical and physical data | |
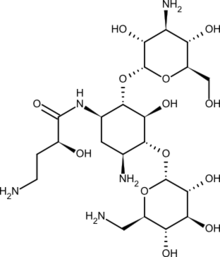
| Formula | C22H43N5O13 |
| Molar mass | 585.603 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amikacin is an antibiotic used for a number of bacterial infections. This includes joint infections, intraabdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections.[1] It is also used for the treatment of multidrug-resistant tuberculosis.[2] It is used either by injection into a vein or muscle.[1]
Common side effects include hearing loss, balance problems, and kidney problems. Other side effects include paralysis resulting in the inability to breathe. If used during pregnancy it may cause permanent deafness in the baby. Amikacin is in the aminoglycoside family of medications. It works by blocking the function of the bacteria's 30S ribosomal subunit, making it unable to make protein.[1]
Amikacin was patented in 1971 and came into commercial use in 1976.[3][4] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[5] The wholesale cost in the developing world is 13.80 to 130.50 USD for a month.[6] In the United States a typical course of treatment costs 25 to 50 USD.[7] It is made from kanamycin.[1]
Medical uses
Amikacin is most often used for treating severe, hospital-acquired infections with multidrug-resistant Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter, and Enterobacter. Serratia marcescens and Providencia stuartii are also included in the spectrum. Amikacin can also be used to treat non-tubercular mycobacterial infections and tuberculosis (if caused by sensitive strains) when first-line drugs fail to control the infection.
Amikacin may be combined with a beta-lactam antibiotic for empiric therapy for people with neutropenia and fever.
Liposomal amikacin for inhalation is currently in late stage clinical trials for the treatment of respiratory diseases, such as cystic fibrosis,[8] Pseudomonas aeruginosa,[9] non-tubercular mycobacterial infections[10] and bronchiectasis.[11][12]
Bacterial susceptibility data
Amikacin is usually used as a last-resort medication against multidrug-resistant bacteria. The following represents susceptibility data on a few medically significant microorganisms.
- Pseudomonas aeruginosa0.5 μg/mL – 32 μg/mL
- Pseudomonas aeruginosa (aminoglycoside-resistant) – 32 μg/mL – 64 μg/mL
- Serratia marcescens – ≤0.25 μg/mL – 8 μg/mL
- Serratia marcescens (multidrug-resistant) – 32 μg/mL
Adverse effects
Side-effects of amikacin are similar to those of other aminoglycosides. Kidney damage and hearing loss are the most important effects. Because of this potential, blood levels of the drug and markers of kidney function (creatinine) may be monitored. Moreover, doses are adjusted specifically based upon serum Creatinine clearance in clinical settings.
Administration
Amikacin may be administered once or twice a day but must be given by the intravenous or intramuscular route or via nebulization. There is no oral form available as amikacin is not absorbed orally. In people with kidney failure, dosage must be adjusted according to the creatinine clearance, usually by reducing the dosing frequency.
Resistance
Amikacin evades attacks by most of the antibiotic-inactivating enzymes that are responsible for antibiotic resistance in bacteria. This is accomplished by the L-hydroxyaminobuteroyl amide (L-HABA) moiety attached to N-1 (compare to kanamycin), which inhibits acetylation, phosphorylation, and adenylation in the distant amino sugar ring (C-2,C-3,C-4). To prevent the development of bacterial resistance to this antibiotic, its use is tightly regulated.
References
- 1 2 3 4 "Amikacin Sulfate". The American Society of Health-System Pharmacists. Retrieved 8 December 2016.
- ↑ WHO Model Formulary 2008 (PDF). World Health Organization. 2009. p. 137. ISBN 9789241547659. Retrieved 8 December 2016.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 507. ISBN 9783527607495.
- ↑ Oxford Handbook of Infectious Diseases and Microbiology. OUP Oxford. 2009. p. 56. ISBN 9780191039621.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- ↑ "Amikacin Sulfate". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 35. ISBN 9781284057560.
- ↑ "Randomized, open-label, active-controlled, multicenter study to assess the efficacy, safety and tolerability of Arikace™ in Cystic Fibrosis patients with chronic infection due to Pseudomonas aeruginosa" is a European Phase III clinical trial, being conducted across multiple sites in the EU, starting at the Royal Brompton Hospital, Department of Respiratory Medicine, in London. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-000441-20/GB
- ↑ http://www.clinicaltrials.gov/ct2/show/NCT01315678
- ↑ "A Randomized, Double-Blind, Placebo-Controlled Study of Liposomal Amikacin for Inhalation (Arikace™) in Patients With Recalcitrant Nontuberculous Mycobacterial Lung Disease" is a Phase II clinical trial in collaboration with the US National Institute of Allergy and Infectious Diseases. http://www.clinicaltrials.gov/ct2/show/NCT01315236
- ↑ "A Placebo Controlled, Randomized, Parallel Cohort, Safety And Tolerability Study Of 2 Dose Levels Of Liposomal Amikacin For Inhalation (Arikace™) In Patients With Bronchiectasis Complicated By Chronic Infection Due To Pseudomonas Aeruginosa" Phase II (completed). http://www.clinicaltrials.gov/ct2/show/NCT00775138
- ↑ "A Study to Determine the Safety and Tolerability of Arikace™ Versus Placebo in Patients Who Have Bronchiectasis" is a Phase II clinical trial (as [4]) completed in the UK. http://www.ukctg.nihr.ac.uk/trialdetails/NCT00775138
- ↑ http://www.toku-e.com/Assets/MIC/Amikacin%20hydrate.pdf
- Edson RS, Terrell CL. The aminoglycosides. Mayo Clin Proc. 1999 May;74(5):519–28. Review. PMID 10319086