Aluminosilicate
Aluminosilicate minerals are minerals composed of aluminium, silicon, and oxygen, plus countercations. They are a major component of kaolin and other clay minerals.
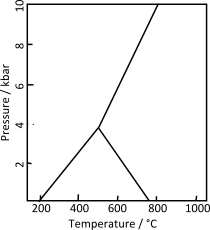
Andalusite, kyanite, and sillimanite are naturally occurring aluminosilicate minerals that have the composition Al2SiO5.[3][4][5] The triple point of the three polymorphs is located at a temperature of 500 °C and a pressure of 0.4 GPa. These three minerals are commonly used as index minerals in metamorphic rocks.
Hydrated aluminosilicate minerals are referred to as zeolites and are porous structures that are naturally occurring materials.
The catalyst silica-alumina is an amorphous substance which is not an aluminosilicate compound.
Aluminosilicate glasses
There exist a wide variety of glass types. The characteristics of these different types depend on the chemical composition of the glass with a special focus on the oxide composition. Glasses can be categorized into many different groups, one of them including the so-called aluminosilicate glasses. This glass type can be further differentiated:
Alkaline earth aluminosilicate glasses
Characteristically, these glasses are free of alkali oxides and contain 15-25% AI2O3, 52-60% SiO2 and about 15% alkaline earths. Very high transformation temperatures and softening points are typical features. Main fields of application are glass bulbs for halogen lamps, high-temperature thermometers and thermally and electrically highly loadable film resistors.
Alkali aluminosilicate glasses
The AI2O3 content of alkali aluminosilicate glasses is typically 10-25% and the alkali content over 10%. The high alkali content prepares the glass for ion exchange with bigger alkali ions in order to improve the surface compressive strength. Due to this particular feature, this glass type is especially suitable for the use in touch displays, solar cells cover glass and laminated safety glass. High transformation temperatures and outstanding mechanical properties, e.g. hardness and scratch behavior, are characteristic of this glass type.[6]
See also
- Aluminium silicate
- Silicate minerals
- Calcium aluminosilicate
- Sodium aluminosilicate
- Gorilla Glass - a type of aluminosilicate glass
References
- ↑ Whitney, D.L. (2002), "Coexisting andalusite, kyanite, and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point, Sivrihisar, Turkey", American Mineralogist, 87 (4): 405–416
- ↑ Whitney, D.L. (2002), "Coexisting andalusite, kyanite, and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point, Sivrihisar, Turkey", American Mineralogist, 87 (4): 405–416
- ↑ Andalusite, Handbook of Mineralogy
- ↑ Kyanite, Handbook of Mineralogy
- ↑ Sillimanite, Handbook of Mineralogy
- ↑ Technical Glasses