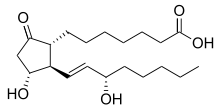
Prostaglandin E1
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Caverject, Muse, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695022 |
| Pregnancy category |
|
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.010.925 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.481 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Prostaglandin E1 (PGE1), also known as alprostadil, is a naturally occurring prostaglandin which is used as a medication.[1] In babies with congenital heart defects, it is used by slow injection into a vein to open the ductus arteriosus until surgery can be carried out.[2] By injection into the penis or placement in the urethra, it is used to treat erectile dysfunction.[3]
Common side effects when given to babies include decreased breathing, fever, and low blood pressure. When used for erectile dysfunction side effects may include penile pain, bleeding at the site of injection, and prolonged erection. Prostaglandin E1 is in the vasodilator family of medications. It works by opening blood vessels by relaxing smooth muscle.[1]
Prostaglandin E1 was isolated in 1957 and approved for medical use in the United States in 1981.[1][4] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[5] In the United Kingdom a dose costs the NHS about 75 pounds.[6] In the United States it costs 100 to 200 USD per dose.[7] Prostaglandin E2 works as well as prostaglandin E1 in babies; however, is much less expensive.[2]
Medical uses
Patent ductus arteriosus
Alprostadil is also used in maintaining a patent ductus arteriosus in newborns. This is primarily useful when the threat of premature closure of the ductus arteriosus exists in an infant with ductal-dependent congenital heart disease, including cyanotic lesions (e.g., hypoplastic left heart syndrome, pulmonary atresia/stenosis, tricuspid atresia/stenosis, transposition of the great arteries) and acyanotic lesions (e.g., coarctation of the aorta, critical aortic stenosis, and interrupted aortic arch).
Sexual dysfunction
Alprostadil is sold in the United States as urethral suppositories and in injectable form. The suppositories are sold under the brand name Muse.[8] The injectable forms are Edex[9] and Caverject.[10] Muse delivers alprostadil as a penile suppository, inserted into the urethra, at least ten minutes before the erection is needed. Caverject and Edex are similarly fast-acting, but instead are injected by syringe directly into the corpus cavernosum of the penis.
Alprostadil is also available as a generic. The major cost is that it must be mixed by a compounding pharmacy and supplies may be difficult to obtain. The different formulations, including Bimix and Trimix, may include papaverine and/or phentolamine. A typical mix might be 30 mg of papaverine, 2 mg of phentolamine, and 20 μg alprostadil. As a generic, it is much less expensive than the packaged injectables. It is premixed and must be kept refrigerated and the user must load a syringe with the quantity needed.
Most recently, the compound has been made easily accessible in an applicable topical cream form known as Vitaros.[11] Made by Takeda UK Ltd, it is now available in Europe and contains either 200 or 300 micrograms of alprostadil in 100 mg of cream which is directly administered as a topical cream applied to the urethra in a preloaded delivery device. The tip of the device is placed in the urethral meatus and the cream delivered into the urethra. Clinical trials for the treatment showed positive results in over 3000 men that it was tested on, and unlike other sexual dysfunction medication, it is said to be usable by men suffering from diabetes or heart problems and those who have undergone a prostatectomy.[12] It has no known interactions with food, alcohol or other medications making it safer than other treatments containing alprostadil. Similarly to the Bimix and Trimix injections though, it must be kept under cool temperatures.
Critical limb ischemia
Alprostadil is also used for critical limb ischemia. It increases blood flow by peripheral vasodilation within five minutes and induces angiogenesis. It is most effective when the ankle pressure is at least 30 mmHg and at least one tibial artery is patent.
Adverse effects
- Accidental injury (Muse only)
- Apnea
- Bleeding:
- Cerebral
- Urethral
- Bradycardia
- Cardiac arrest
- Congestive heart failure
- Cortical proliferation of long bones
- Diarrhea
- Disseminated intravascular coagulation
- Edema
- Fever
- Flushing
- Hyperemia
- Hypotension
- Injection-site haematoma
- Injection-site ecchymosis (Caverject only)
- Pain:
- Back
- Pelvic
- Penile
- Testicular (Muse only)
- Urethral
- Prolonged erection
- Penile fibrosis
- Second-degree heart block
- Seizures
- Sepsis
- Shock
- Spasm of right ventricle infundibulum
- Supraventricular tachycardia
- Tachycardia
- Ventricular fibrillation
- Urethral burning
- Uterine rupture
Biosynthesis
Prostaglandin E1 is biosynthesized on an as-needed basis from dihomo-γ-linolenic acid (an omega-6 fatty acid) in healthy humans without coronary artery disease[13] and/or a genetic disorder.
Other versions
Misoprostol is another synthetic prostaglandin E1 analog used to prevent gastric ulcers when taken on a continuous basis, to treat missed miscarriage, to induce labor, and to induce abortion.
References
- 1 2 3 "Alprostadil". The American Society of Health-System Pharmacists. Retrieved 8 January 2017.
- 1 2 Northern Neonatal Network (208). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (5 ed.). John Wiley & Sons. p. 2010. ISBN 9780470750353.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 569. ISBN 9780857111562.
- ↑ Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 185. ISBN 9780470015520.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- ↑ Ainsworth, Sean B. (2014). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life. John Wiley & Sons. p. 436. ISBN 9781118819593.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 477. ISBN 9781284057560.
- ↑ "Muse Suppository - Facts and Comparisons". Drugs.com. Retrieved 4 January 2013.
- ↑ Edex - Facts and Comparisons Drugs.com
- ↑ Caverject - Facts and Comparisons Drugs.com
- ↑ Vitaros 3 mg/g cream - Summary of Product Characteristics Medicines.org.uk
- ↑ Vitaros- New Erectile Dysfunction Topical Treatment Meds4All.co.uk
- ↑ Stephanie M. Meller, BA, Erik Stilp, MD, Charles N. Walker, MD, Carlos Mena-Hurtado, MD (June 2013). "The Link Between Vasculogenic Erectile Dysfunction, Coronary Artery Disease, and Peripheral Artery Disease: Role of Metabolic Factors and Endovascular Therapy". Journal of Invasive Cardiology. 25 (6): 313–319.