Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.[1]
Organopalladium chemistry timeline
- 1873 - A. N. Zaitsev reports reduction of benzophenone over palladium with hydrogen.
- 1894 - Phillips reports that palladium(II) chloride reduces to palladium metal by contact with ethylene.[2]
- 1907 - Autoclave technology introduced by W. Ignatieff makes it possible to carry out high pressure hydrogenation.
- 1956 - In the Wacker process ethylene and oxygen react to acetaldehyde with catalyst PdCl2/CuCl2
- 1972 - The Heck reaction is a coupling reaction of a halogenide with an olefin. The catalyst is Pd(0).
- 1973 - The Trost asymmetric allylic alkylation is a nucleophilic substitution.
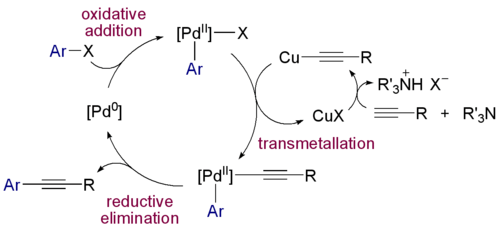
- 1975 - The Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides.
Overview
In contrast to its next-door neighbors the group 11 elements, the element palladium in organic chemistry does not involve preparation of organopalladium compounds itself but rather organopalladium reactive intermediates.[3] On top of that in many reactions only catalytical amounts of the metal are used.
Palladium alkene complexes
Palladium reacts with alkenes to form a pi complex which can react with a multitude of nucleophiles akin a oxymercuration reaction. The C-Pd bond is then removed by a reduction or an elimination. In the industrially important Wacker process, ethylene is converted to acetaldehyde with palladium chloride. Fullerene ligands also bind with palladium in similar ways as ethylene.
Palladium allyl complexes
Allyl compounds with suitable leaving groups react with palladium(II) salts to pi-allyl complexes having hapticity 3 such as the allylpalladium chloride dimer. These intermediates too react with nucleophiles for example carbanions derived from malonates [4] or with amines in allylic amination [5] as depicted below [6]
Allylpalladium intermediates also feature in the Trost asymmetric allylic alkylation and the Carroll rearrangement and an oxo variation in the Saegusa oxidation.
Palladium-carbon sigma-bonded complexes
Various organic groups can bound to palladium and form stable sigma-bonded complexes. Currently, the alkyl, vinyl, aryl, and alkynyl complexes with Pd-C(sp3), Pd-C(sp2), Pd-C(sp) bonds are equally well-known. The stability of the bonds in terms of bond dissociation energy follows the trend: Pd-Alkynyl > Pd-Vinyl ≈ Pd-Aryl > Pd-Alkyl and the metal-carbon bond length changes in the opposite direction: Pd-Alkynyl < Pd-Vinyl ≈ Pd-Aryl < Pd-Alkyl.[7]
Palladium insertion compounds
Zerovalent Pd(0) compounds such as tris(dibenzylideneacetone)dipalladium(0) and tetrakis(triphenylphosphine)palladium(0) react with halocarbon R-X in oxidative addition to R-Pd-X intermediates with covalent Pd-C bonds. This chemistry forms the basis of a large class of organic reactions called coupling reactions (see palladium-catalyzed coupling reactions). An example is the Sonogashira reaction:
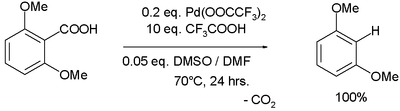
Palladium(II) trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:[8]
In the proposed reaction mechanism Pd(II) replaces the carboxylic acid proton while losing a TFA group, carbon dioxide is lost in a first-order reaction and TFA destroys the formed Ar-Pd-TFA complex without Pd changing its oxidation state.
Organopalladium(IV)
The first organopalladium(IV) compound was described in 1986. This complex is Me3Pd(IV)Ibpy with bpy as a bidentate 2,2'-bipyridine ligand [9] It was synthesized by reaction of methyl iodide with Me2Pd(II)bpy.
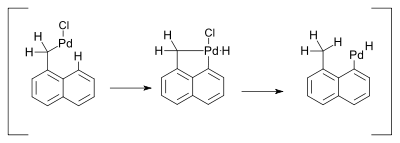
Palladium compounds owe their reactivity to the ease of interconversion between Pd(0) and palladium(II) intermediates. There is no conclusive evidence however for the involvement of Pd(II) to Pd(IV) conversions in palladium mediated organometallic reactions.[10] One reaction invoking such mechanism was described in 2000 and concerned a Heck reaction. This reaction was accompanied by a 1,5-hydrogen shift in the presence of amines:[11]
The hydride shift was envisaged as taking place through a Pd(IV) metallacycle:
In related work the intermediate associated with the hydride shift remains Pd(II):[12]
and in other work (a novel synthesis of indoles with two Pd migrations) equilibria are postulated between different palladacycles:[13][14]
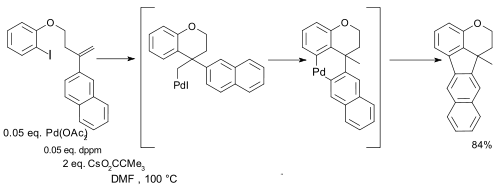
and in certain intramolecular couplings synthetic value was demonstrated regardless of oxidation state:[15]
See also
- Compounds of carbon with other elements in the periodic table:
| Compounds of carbon with other elements in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ Handbook of Organopalladium Chemistry for Organic Synthesis Ei-Negishi John Wiley (2002) ISBN 0-471-31506-0
- ↑ Phillips, F. C.; Am. Chem. J. 1894, 16, 255.
- ↑ F.A. Carey R.J. Sundberg Advanced Organic Chemistry 2nd Ed. ISBN 0-306-41199-7
- ↑ Jan-E. Bäckvall and Jan O. Vågberg (1993). "Stereoselective 1,4-Functionalizations of Conjugated Dienes: cis- and trans-1-Acetoxy-4-(Dicarbomethoxymethyl)-2-Cyclohexene". Org. Synth.; Coll. Vol., 8, p. 5
- ↑ Igor Dubovyk; Iain D. G. Watson & Andrei K. Yudin (2007). "Chasing the Proton Culprit from Palladium-Catalyzed Allylic Amination". J. Am. Chem. Soc. 129 (46): 14172–14173. PMID 17960935. doi:10.1021/ja076659n.
- ↑ Reagents: triethyl phosphite ligand, DBU (is reported to absorb the amine protons that would otherwise trigger isomerization) in THF
- ↑ V. P. Ananikov et al., Organometallics, 2005, 24, 715. doi:10.1021/om0490841
- ↑ Joshua S. Dickstein; Carol A. Mulrooney; Erin M. O'Brien; Barbara J. Morgan & Marisa C. Kozlowski (2007). "Development of a Catalytic Aromatic Decarboxylation Reaction". Org. Lett. 9 (13): 2441–2444. PMID 17542594. doi:10.1021/ol070749f.
- ↑ Peter K. Byers; Allan J. Canty; Brian W. Skelton; Allan H. White (1986). "The oxidative addition of lodomethane to [PdMe2(bpy)] and the X-ray structure of the organopalladium(IV) product fac-[PdMe3(bpy)l](bpy = 2,2-bipyridyl)". Chem. Commun. (23): 1722–1724. doi:10.1039/C39860001722.
- ↑ Antonio J. Mota & Alain Dedieu (2007). "Through-Space Intramolecular Palladium Rearrangement in Substituted Aryl Complexes: Theoretical Study of the Aryl to Alkylpalladium Migration Process". J. Org. Chem. 72 (25): 9669–9678. PMID 18001098. doi:10.1021/jo701701s.
- ↑ Liansheng Wang; Yi Pan; Xin Jiang; Hongwen Hu (2000). "Palladium catalyzed reaction of α-chloromethylnaphthalene with olefins". Tetrahedron Letters. 41 (5): 725–727. doi:10.1016/S0040-4039(99)02154-1.
- ↑ C-H Activation and Palladium Migration within Biaryls under Heck Reaction Conditions Gunter Karig, Maria-Teresa Moon, Nopporn Thasana, and Timothy Gallagher Org. Lett., Vol. 4, No. 18, 2002 3116 doi:10.1021/ol026426v
- ↑ Synthesis of Substituted Carbazoles by a Vinylic to Aryl Palladium Migration Involving Domino C-H Activation Processes Jian Zhao and Richard C. Larock Org. Lett., Vol. 7, No. 4, 701 2005 doi:10.1021/ol0474655
- ↑ Reagents: diphenylacetylene, palladium acetate, bis(diphenylphosphino)methane (dppm) and the caesium salt of pivalic acid (CsPiv)
- ↑ Pd-Catalyzed Alkyl to Aryl Migration and Cyclization: An Efficient Synthesis of Fused Polycycles via Multiple C-H Activation Qinhua Huang, Alessia Fazio, Guangxiu Dai, Marino A. Campo, and Richard C. Larock J. AM. CHEM. SOC. 2004, 126, 7460-7461 doi:10.1021/ja047980y