Alkenylsuccinic anhydride
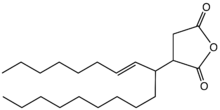
An alkenylsuccinic anhydride (ASA) is a type of organic compound that is related to succinic anhydride, but with an alkenyl group replacing one hydrogen. For most applications, the alkenyl group is C18 chain since it is intended to confer hydrophobicity. They are usually colorless viscous liquids.[1]
ASAs have a variety of commercial applications. They are used as sizing agents in the paper industry. In this role, the anhydride is proposed to form an ester with the hydroxyl groups on the cellulose fibers and the alkenyl chain modifies the surface properties of the paper product.[2] The application is similar to that for alkyl ketene dimers.
Preparation
ASAs are prepared by heating maleic anhydride with olefins to around 200°C. An Alder-ene reaction occurs, giving a mixture of isomers.[3]
Polyisobutenylsuccinic anhydride
ASAs are related to the class of polyisobutylenylsuccinic anhydrides, known as PIBSAs. In these compounds, the alkene used is polyisobutylene. Such compounds are commonly used as reactive intermediates in the petroleum additive industry. They are reacted with ethyleneamines to give the corresponding succinimides useful as dispersants[4] and deposit-control agents[5] in lubricants and fuels.
There are two types of polyisobutylene used for this purpose, and they are commonly known as conventional and HR (highly reactive) PIB. Conventional PIB is made by polymerizing Raffinate 1 that contains a mixture of C4 butenes with aluminum trichloride as catalyst. In comparison, HR PIB is made by polymerizing isobutylene using boron trifluoride as catalyst. The methyl vinylidene content of PIB controls its reactivity toward maleic anhydride. HR PIB has 85 % methyl vinylidene, making it more reactive than conventional PIB that only has 10 %. Being more reactive, HR PIB requires less forcing conditions for its reaction with maleic anhydride. The PIBSA produced contains less tars and side products as a result.[6]
References
- ↑ Werner J. Auhorn "Paper and Board, 3. Chemical Additives" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim. 2012. doi:10.1002/14356007.o18_o11
- ↑ Gess, Jerome; Rend, Dominic (2005). "Alkenyl Succinic Anhydride (ASA)". Tappi Journal. 4: 25–30.
- ↑ Ramaswamy, R.; Achary, P. Sasidharan; Shine, K. G. (1987). "Some aspects on the synthesis and characterization of dodecenyl succinic anhydride (DDSA)—a curing agent for epoxy resins". Journal of Applied Polymer Science. 33: 49. doi:10.1002/app.1987.070330105.
- ↑ "Polyisobutylene Succinimides in Engine Oil". Lubrizol. Retrieved 2017-02-14.
- ↑ J. Reid and J. Barker (2013). "SAE Technical Paper Series". SAE Technical Paper Series. 1. doi:10.4271/2013-01-2682.
|chapter=ignored (help) - ↑ Mach, H.; Rath, P. (1999). "Highly reactive polyisobutene as a component of a new generation of lubricant and fuel additives". Lubrication Science. 11 (2): 175. doi:10.1002/ls.3010110205.