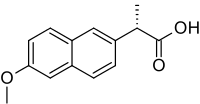
Naproxen
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /nəˈprɒksən/ |
| Trade names | Aleve, Naprosyn, Anaprox, Naprelan, Flanax, Aflaxen, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681029 |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% (oral) |
| Protein binding | 99% |
| Metabolism | Hepatic (to 6-desmethylnaproxen) |
| Biological half-life | 12-17 hours (adults)[4] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.040.747 |
| Chemical and physical data | |
| Formula | C14H14O3 |
| Molar mass | 230.259 g/mol |
| 3D model (JSmol) | |
| Melting point | 152–154 °C (306–309 °F) |
| |
| (verify) | |
Naproxen (brand names: Aleve, Naprosyn, and many others) is a nonsteroidal anti-inflammatory drug (NSAID) of the propionic acid class (the same class as ibuprofen) that relieves pain, fever, swelling, and stiffness.[6][7] It is a nonselective COX inhibitor, usually sold as the sodium salt.
Naproxen poses an intermediate risk of stomach ulcers compared with ibuprofen, which is low-risk, and indometacin, which is high-risk.[8] To reduce stomach ulceration risk, it is often combined with a proton-pump inhibitor (a medication that reduces stomach acid production) during long-term treatment of those with pre-existing stomach ulcers or a history of developing stomach ulcers while on NSAIDs.[6][7]
Medical uses

Naproxen's medical uses are related to its mechanism of action as an anti-inflammatory compound. Naproxen is used to treat a variety of inflammatory conditions and symptoms that are due to excessive inflammation, such as pain and fever (naproxen has fever-reducing, or antipyretic, properties in addition to its anti-inflammatory activity). Notably, not all medications that reduce fever are anti-inflammatory compounds (such as paracetamol). Inflammatory sources of pain that may respond to naproxen's anti-inflammatory activity are conditions such as migraine, osteoarthritis, kidney stones, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, menstrual cramps, tendinitis and bursitis.[1] It is also used to treat primary dysmenorrhea.[10]
Because of its anti-inflammatory mechanism of action, one would not expect naproxen to be useful in treating non-inflammatory causes of pain (e.g.,, diabetic nerve pain).
Naproxen is used as a "bridge therapy" in medication-overuse headache to wean patients off other medications.[11]
Diagnostics
Naproxen has been used to differentiate between infectious fevers and neoplastic or connective tissue disease-related fevers.[12] Although the literature is inconclusive, it is thought that naproxen may help differentiate between infectious fevers and neoplastic fevers by its efficacy in reducing them; in some studies, naproxen reduced neoplastic fevers far better than it reduced infectious fevers. This information could potentially be used to identify the etiology of the patient's fever, which can be complex in cancer patients (whom are often at heightened risk for infection in the first place).[13]
Adverse effects
Naproxen should be taken orally with food to decrease the risk of gastrointestinal side effects. Common adverse effects include dizziness, drowsiness, headache, rash, bruising, and gastrointestinal upset.[1]
In the U.S., naproxen is sold with boxed warnings about the risk of gastrointestinal ulceration or bleeding.[1]
COX-2 selective and nonselective NSAIDs have been linked to increases in the number of serious and potentially fatal cardiovascular events, such as myocardial infarctions and strokes.[14] Naproxen is, however, associated with the smallest overall cardiovascular risks.[15][16] Cardiovascular risk must be considered when prescribing any nonsteroidal anti-inflammatory drug. The drug had roughly 50% of the associated risk of stroke compared with ibuprofen, and was also associated with a reduced number of myocardial infarctions compared with control groups.[15] As with other non-COX-2 selective NSAIDs, naproxen can cause gastrointestinal problems, such as heartburn, constipation, diarrhea, ulcers and stomach bleeding.[17] Persons with a history of ulcers or inflammatory bowel disease should consult a doctor before taking naproxen.
A study found that high-dose naproxen induced near-complete suppression of platelet thromboxane throughout the dosing interval and appeared not to increase cardiovascular disease (CVD) risk, whereas other non-aspirin high-dose NSAID regimens had only transient effects on platelet COX-1 and were associated with a small but definite vascular hazard. Conversely, naproxen was associated with higher rates of upper gastrointestinal bleeding complications compared with other NSAIDs.[16]
Interactions
Naproxen should not be taken with antidepressants, lithium, methotrexate, probenecid, a blood thinner, heart or blood pressure medications, including diuretics or steroid medicine (such as prednisone).[1]
NSAIDs such as naproxen may interfere with and reduce the efficacy of SSRI antidepressants,[18] as well as increase the risk of bleeding greater than the individual bleeding risk of either class of agent when taken together.[19] Naproxen is not contraindicated in the presence of SSRIs, though concomitant use of the medications should be done with caution.[19]
Alcohol consumption increases the risk of gastrointestinal bleeding when combined with naproxen in a dose-dependent manner (that is, the higher the dose of naproxen, the higher the risk of bleeding).[20]
Pharmacology
Mechanism of action
Naproxen works by reversibly inhibiting both the COX-1 and COX-2 enzymes as a non-selective coxib.[21][22][23][24][25] This results in the inhibition of prostaglandin synthesis. Prostaglandins act as signaling molecules in the body, inducing inflammation. Thus, by inhibiting COX-1/2, naproxen induces an anti-inflammatory effect.
Pharmacokinetics
Naproxen is a minor substrate of CYP1A2 and CYP2C9. It is extensively metabolized in the liver to 6-O-desmethylnaproxen, and both the parent drug and the desmethyl metabolite undergo further metabolism to their respective acylglucuronide conjugated metabolites.[26] An analysis of two clinical trials shows that naproxen's time to peak plasma concentration occurs between 2–4 hours after oral administration, though naproxen sodium reaches peak plasma concentrations within 1–2 hours.[4]
Pharmacogenetics
The pharmacogenetics of naproxen has been studied in an effort to better understand its adverse effects.[27] In 1998, a small pharmacokinetic (PK) study failed to show that differences in a patient's ability to clear naproxen from the body could account for differences in a patient's risk of experiencing the adverse effect of a serious gastrointestinal bleed while taking naproxen.[27] However, the study failed to account for differences in the activity of CYP2C9, an drug metabolizing enzyme responsible for clearing naproxen.[27] Studies on the relationship between CYP2C9 genotype and NSAID-induced gastrointestinal bleeds have shown that genetic variants in CYP2C9 that reduce the clearance of major CYP2C9 substrates (like naproxen) increase the risk of NSAID-induced gastrointestinal bleeds, especially for homozygous defective variants.[27]
As of April 2017, there are no recommendations for routine CYP2C9 testing for naproxen.[28]
Pregnancy and lactation
Small amounts of naproxen are excreted in breast milk.[1] However, adverse effects are uncommon in infants breastfed from mother taking naproxen.[29]
Chemistry
Naproxen is a member of the 2-arylpropionic acid (profen) family of NSAIDs.[30] The free acid is an odorless, white to off-white crystalline substance. It is lipid-soluble and practically insoluble in water. It has a melting point of 152–155 °C.
Marketing and brand names
Naproxen and naproxen sodium are marketed under various brand names, including: Aleve, Accord, Anaprox, Antalgin, Apranax, Feminax Ultra, Flanax, Inza, Maxidol, Midol Extended Relief, Nalgesin, Naposin, Naprelan, Naprogesic, Naprosyn, Narocin, Pronaxen, Proxen, Soproxen, Synflex, MotriMax, and Xenobid. It is also available bundled with esomeprazole magnesium in delayed release tablets under the brand name Vimovo.[31]
Access restrictions
Syntex first marketed naproxen in 1976 as the prescription drug Naprosyn. They first marketed naproxen sodium under the brand name Anaprox in 1980. It remains a prescription-only drug in much of the world. In the United States, the Food and Drug Administration (FDA) approved it as an over-the-counter (OTC) drug in 1994. OTC preparations in the U.S. are mainly marketed by Bayer HealthCare under the brand name Aleve and generic store brand formulations in 220 mg tablets. In Australia, packets of 275 mg tablets of naproxen sodium are Schedule 2 pharmacy medicines, with a maximum daily dose of five tablets or 1375 mg. In the United Kingdom, 250 mg tablets of naproxen were approved for OTC sale under the brand name Feminax Ultra in 2008, for the treatment of primary dysmenorrhoea in women aged 15 to 50.[32] In the Netherlands, 220 mg and 275 mg tablets are available OTC in drugstores, 550 mg is OTC only at pharmacies. Aleve became available over-the-counter in most provinces in Canada on 14 July 2009, but not British Columbia, Quebec or Newfoundland and Labrador;[33] it subsequently became available OTC in British Columbia in January 2010.[34]
Research
Naproxen may have antiviral activity against influenza. Specifically, it blocks the RNA-binding groove of the nucleoprotein of the virus, preventing formation of the ribonucleoprotein complex—thus taking the viral nucleoproteins out of circulation.[35]
Use in horses
Naproxen is given orally to horses at a dose of 10 mg/kg, and has shown to have a wide safety margin (no toxicity when given at 3-times the recommended dose of 42 days).[36] It is more effective for myositis than the commonly used NSAID phenylbutazone, and has shown especially good results for treatment of equine exertional rhabdomyolysis,[37] a disease of muscle breakdown, but is less commonly used for musculoskeletal disease.
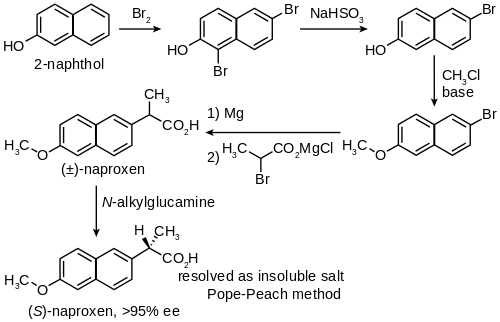
Synthesis
Naproxen has been industrially produced by Syntex starting from 2-naphthol as follows:[38]
References
- 1 2 3 4 5 6 "Naproxen". Drugs.com. 2017. Retrieved 7 February 2017.
- ↑ Gill, A, ed. (July 2013). Standard for the Uniform Scheduling of Medicines and Poisons No. 4 (PDF). The Poisons Standard 2013. Therapeutic Goods Administration. ISBN 978-1-74241-895-7.
- ↑ "NAPROXEN". British National Formulary.
- 1 2 Angiolillo, Dominick J.; Weisman, Steven M. (November 8, 2016). "Clinical Pharmacology and Cardiovascular Safety of Naproxen". Am J Cardiovasc Drugs. 17 (2): 97–107. PMC 5340840
 . doi:10.1007/s40256-016-0200-5.
. doi:10.1007/s40256-016-0200-5. - ↑ https://pubchem.ncbi.nlm.nih.gov/compound/naproxen
- 1 2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 665, 673. ISBN 978-0-85711-084-8.
- ↑ Richy, F; Bruyere, O; Ethgen, O; Rabenda, V; Bouvenot, G; Audran, M; Herrero-Beaumont, G; Moore, A; Eliakim, R; Haim, M; Reginster, JY (July 2004). "Time dependent risk of gastrointestinal complications induced by nonsteroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach.". Annals of the Rheumatic Diseases. 63 (7): 759–66. PMC 1755051
 . PMID 15194568. doi:10.1136/ard.2003.015925.
. PMID 15194568. doi:10.1136/ard.2003.015925. - ↑ "L490 (Naproxen 220 mg)". drugs.com. Drugs.com. Retrieved 17 May 2017.
- ↑ French L (2005). "Dysmenorrhea" (PDF). Am Fam Physician. 71 (2): 285–91. PMID 15686299.
- ↑ Garza, Ivan; Schwedt, Todd J. "Diagnosis and Management of Chronic Daily Headache". medscape.com. WebMD LLC. Retrieved 17 May 2017.
- ↑ Zell JA, Chang JC (November 2005). "Neoplastic fever: a neglected paraneoplastic syndrome". Support Care Cancer. 13 (11): 870–7. PMID 15864658. doi:10.1007/s00520-005-0825-4.
- ↑ Kudlowitz, David. "Neoplastic Fever: Pathophysiology, Clinical Features, And Diagnostic Assessment". Clinical Correlations. New York University. Retrieved 4 May 2017.
- ↑ Nissen, Steven E.; Yeomans, Neville D.; Solomon, Daniel H.; Lüscher, Thomas F.; Libby, Peter; Husni, M. Elaine; Graham, David Y.; Borer, Jeffrey S.; Wisniewski, Lisa M.; Wolski, Katherine E.; Wang, Qiuqing; Menon, Venu; Ruschitzka, Frank; Gaffney, Michael; Beckerman, Bruce; Berger, Manuela F.; Bao, Weihang; Lincoff, A. Michael (2016). "Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis". New England Journal of Medicine. 375 (26): 2519–2529. ISSN 0028-4793. doi:10.1056/NEJMoa1611593.
- 1 2 Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P (2011). "Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis". BMJ. 342: c7086. PMC 3019238
 . PMID 21224324. doi:10.1136/bmj.c7086. c7086.
. PMID 21224324. doi:10.1136/bmj.c7086. c7086. - 1 2 Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C (August 2013). "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials". Lancet. 382 (9894): 769–79. PMC 3778977
 . PMID 23726390. doi:10.1016/S0140-6736(13)60900-9.
. PMID 23726390. doi:10.1016/S0140-6736(13)60900-9. - ↑ Naproxen. PubMed Health.
- ↑ Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P (May 2011). "Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans". Proc. Natl. Acad. Sci. U.S.A. 108 (22): 9262–7. Bibcode:2011PNAS..108.9262W. PMC 3107316
 . PMID 21518864. doi:10.1073/pnas.1104836108.
. PMID 21518864. doi:10.1073/pnas.1104836108. - 1 2 Turner, MS; May, DB; Arthur, RR; Xiong, GL (March 2007). "Clinical impact of selective serotonin reuptake inhibitors therapy with bleeding risks.". J Intern Med. 261 (3): 205–213. PMID 17305643. doi:10.1111/j.1365-2796.2006.01720.x. Retrieved 17 May 2017.
- ↑ Moore, Nicholas; Pollack, Charles; Butkerait, Paul (July 15, 2015). "Adverse drug reactions and drug–drug interactions with over-the-counter NSAIDs". Ther Clin Risk Manag. 11: 1061–1075. PMC 4508078
 . doi:10.2147/TCRM.S79135.
. doi:10.2147/TCRM.S79135. - ↑ Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ (November 2010). "Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen". J. Biol. Chem. 285 (45): 34950–9. PMC 2966109
 . PMID 20810665. doi:10.1074/jbc.M110.162982.
. PMID 20810665. doi:10.1074/jbc.M110.162982. - ↑ Hinz B, Cheremina O, Besz D, Zlotnick S, Brune K (April 2008). "Impact of naproxen sodium at over-the-counter doses on cyclooxygenase isoforms in human volunteers". Int J Clin Pharmacol Ther. 46 (4): 180–6. PMID 18397691. doi:10.5414/CPP46180.
- ↑ Van Hecken A, Schwartz JI, Depré M, De Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, De Schepper PJ (October 2000). "Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers". J Clin Pharmacol. 40 (10): 1109–20. PMID 11028250. doi:10.1177/009127000004001005 (inactive 2017-01-10).
- ↑ Gross GJ, Moore J (July 2004). "Effect of COX-1/COX-2 inhibition versus selective COX-2 inhibition on coronary vasodilator responses to arachidonic acid and acetylcholine". Pharmacology. 71 (3): 135–42. PMID 15161995. doi:10.1159/000077447.
- ↑ Hawkey CJ (October 2001). "COX-1 and COX-2 inhibitors". Best Pract Res Clin Gastroenterol. 15 (5): 801–20. PMID 11566042. doi:10.1053/bega.2001.0236.
- ↑ Vree, Tom; Van Den Biggelaar-Martea, Magdalena; Verwey-Van Wissen, Corrien; Vree, Jeroen; Guelen, Pieter (August 1993). "Pharmacokinetics of naproxen, its metabolite O-desmethylnaproxen, and their acyl glucuronides in humans". Biopharmaceutics & Drug Disposition. 14 (6): 491–502.
- 1 2 3 4 Rodrigues, AD (November 2005). "Impact of CYP2C9 genotype on pharmacokinetics: are all cyclooxygenase inhibitors the same?". Drug Metab Dispos. 33 (11): 1567–75. PMID 16118328. doi:10.1124/dmd.105.006452. Retrieved 17 May 2017.
- ↑ "CYP2C9". pharmgkb.org. PharmGKB. Retrieved 17 May 2017.
- ↑ "LACTMED: NAPROXEN". TOXNET. NIH. Retrieved 21 July 2017.
- ↑ el Mouelhi M, Ruelius HW, Fenselau C, Dulik DM (1987). "Species-dependent enantioselective glucuronidation of three 2-arylpropionic acids. Naproxen, ibuprofen, and benoxaprofen". Drug Metab. Dispos. 15 (6): 767–72. PMID 2893700.
- ↑ "Vimovo's hotsite". vimovo.com. 11 March 2015.
- ↑ "Medicines regulator approves availability of a new OTC medicine for period pain" (Press release). Medicines and Healthcare products Regulatory Agency (MHRA). 1 April 2008. Archived from the original (PDF) on 21 September 2013.
- ↑ "Aleve – Welcome to Canada, Eh!" (PDF) (Press release). Bayer Health Care. 14 July 2009. Retrieved 24 March 2012.
- ↑ "Aleve – Helping British Columbians with Joint and Arthritis Pain Get Back to Doing the Activities They Love". newswire.ca. 28 January 2010.
- ↑ Lejal N, Tarus B, Bouguyon E, Chenavas S, Bertho N, Delmas B, Ruigrok RW, Di Primo C, Slama-Schwok A (May 2013). "Structure-based discovery of the novel antiviral properties of naproxen against the nucleoprotein of influenza A virus". Antimicrob. Agents Chemother. 57 (5): 2231–42. PMC 3632891
 . PMID 23459490. doi:10.1128/AAC.02335-12. Lay summary – EurekAlert!.
. PMID 23459490. doi:10.1128/AAC.02335-12. Lay summary – EurekAlert!. - ↑ McIlwraith CW, Frisbie DD, Kawcak CE. Nonsteroidal Anti-Inflammatory Drugs. Proc. AAEP 2001 (47): 182-187.
- ↑ May SA, Lees P. Nonsteroidal anti-inflammatory drugs. In McIlwraith CW, Trotter GW, eds. Joint disease in the horse. Philadelphia: WB Saunders, 1996;223–237.
- ↑ Harrington PJ, Lodewijk E (1997). "Twenty Years of Naproxen Technology". Org. Process Res. Dev. 1 (1): 72–76. doi:10.1021/op960009e.
External links
| Look up naproxen in Wiktionary, the free dictionary. |
- CID 1302 from PubChem
- EINECS number 244-838-7
- MedlinePlus Information on naproxen
- FDA Drug Prescribing Information on drugs.com
- FDA Statement on Naproxen, released 20 December 2004
- Alzheimer's Disease Anti-Inflammatory Prevention Trial
- Forbes article (expressing the point of view that the risk of heart attack or stroke was overstated)
- Which NSAID for Heart Disease Patients? – Medscape
- U.S. National Library of Medicine: Drug Information Portal – Naproxen
- Aleve Daily Med
- Naproxen bound to proteins in the PDB