Aldose
An aldose, like a ketose, is a monosaccharide (a simple sugar), which have a carbon backbone chain with many alcohol (hydroxy groups) and an aldehyde. They are polar molecules and very soluble in water due to the many -OH groups they possess. Monosaccharides are a type of carbohydrate, organic structures which can be thought of as having the ingredients for many water (H2O) molecules attached to the carbons in the chain, hence the 'hydrate" of carbohydrate. For most organisms, carbohydrates are an important source of energy that is taken in as food, and broken down in a series of processes called metabolism. Aldoses feature prominently in two metabolic regimes, glycolysis which is the break down of sugars, and gluconeogenesis which is the opposite.
Structure
An aldose contains only one aldehyde (−CH=O) group per molecule, whereas a ketose contains a ketone group. The chemical formula takes the form Cn(H2O)n. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms.[1]
Because they have at least one asymmetric[2] carbon center, aldoses with three or more carbon atoms exhibit stereoisomerism. Aldoses containing stereogenic centers can exist in either a D- form or L- form. The determination is made based on the chirality of the penultimate carbon (the second-furthest from the aldehyde), where alcohol groups on the right of the Fischer projection result in D-aldoses, and epimers with alcohols on the left result in L-aldoses. Biological systems tend to recognize D-aldoses more than L-aldoses.
Examples of aldose include glycolaldehyde, glyceraldehyde, erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, talose, and galactose. [2] All of these examples contain one group of aldehyde. They only differ in numbers of carbons in carbon skeleton. All of these complex sugars serve an important role in biochemistry.
An aldose has a carbonyl group at one end of the carbon chain, forming an aldehyde in its linear form. This allows ketoses and aldoses to be chemically differentiated through Seliwanoff's test.[3] In Seliwanoff's test, aldoses tend to react in a slow pace, and produce a light pink color, while ketoses react with resorcinol to produce a dark red color. With different color of production, aldoses can be differentiate from the ketoses. An aldose may isomerize to a ketose through the Lobry-de Bruyn-van Ekenstein transformation. Aldose and ketose, although differ in structures, also perform in different roles. Aldoses tend to isomerise into ketoses.
Nomenclature and Common Aldoses
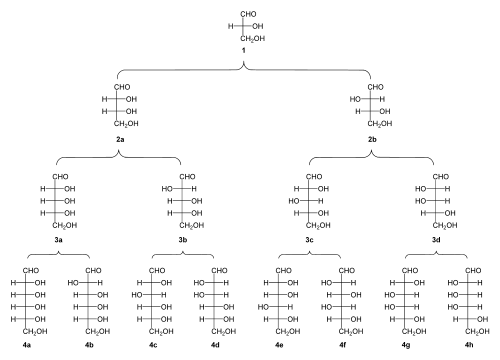
Aldoses, like all carbohydrates are categorized first by the presence of an aldehyde or a ketone on the carbon chain. They can be further differentiated by the number of carbons in the main chain using Greek prefixes. The minimum number of carbons in a backbone needed to form a molecule that is still considered a carbohydrate is 3, and carbohydrates with three carbons are called trioses. The only aldotriose is Glyceraldehyde, which has one chiral stereocenter with 2 possible enantiomers, D and L Glyceraldehyde. The most commonly discussed category of aldoses are those with 6 carbons, aldohexoses. Some aldohexoses that are widely called by common names are
- D-(+)-Talose
to name a few[4]
Aldoses can be further differentiated according to the number of carbon atoms they contain in the main chain using Greek roots (tri-, tet-, pent-, hex-, etc.), the common formula is to place the number signifier after aldo (or keto if it is a ketose), as in aldotriose. The following is a list of other common aldoses.
- Diose: glycolaldehyde
- Triose: glyceraldehyde
- Tetroses: erythrose, threose
- Pentoses: ribose, arabinose, xylose, lyxose
- Hexoses: Glucose
Stereochemistry
Aldoses are commonly referred to by names specific to one stereoisomer of the compound. This distinction is especially vital in Biochemistry, as many systems can only use one enantiomer of the carbohydrate and not the other. However, aldoses are by no means locked into any one conformation forever, they can and do fluctuate between a couple of different forms.
Aldoses can tautomerize to ketoses in a dynamic process with an enol intermediate. This process is reversible and when discussing a mass group of sugars, aldoses and ketoses are thought of as being in equilibrium with each other due to the low energy barrier of the transition. However aldehydes and ketones are almost always more stable than their enol forms, so the aldo- and keto- forms are assumed to predominate at any given moment. This process, with its enol intermediate, when applied occurring at other carbons on the chain allows stereoisomerization. Basic solutions exacerbate the interconversion of isomers.
Carbohydrates, especially those with more than 4 carbons, exist in an equibrium between two forms, often called the cyclic, and open-chain forms for clarity. Cyclic aldoses are usually drawn as Haworth projections, and open chain forms are commonly drawn as Fischer projections, both of which represent important stereochemical information about the forms they depict.
See also
References
- ↑ Berg, J.M. (2006). Biochemistry (6th ed.). New York: W. H. Freeman and Company.
- 1 2 "Difference Between Aldose and Ketose". Difference Between. Retrieved 2016-03-28.
- ↑ "Seliwanoff's Test". Harper College. Retrieved 2011-07-10.
- ↑ Solomons, T.W. Graham (2008). Organic Chemistry. John Wiley & Sons Inc. p. 1044.
