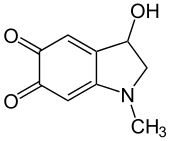
Adrenochrome
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-1-methyl-2,3-dihydro-1H-indole-5,6-dione | |
| Other names
Adraxone; Pink adrenaline | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.176 |
| PubChem CID |
|
| |
| |
| Properties | |
| C9H9NO3 | |
| Molar mass | 179.18 g·mol−1 |
| Density | 3.264 g/cm³ |
| Boiling point | (decomposes, 115-120 °C) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Adrenochrome is a chemical compound with the molecular formula C9H9NO3 produced by the oxidation of adrenaline (epinephrine). The derivative carbazochrome is a hemostatic medication. Despite a similarity in chemical names, it is unrelated to chrome or chromium.
Chemistry
In vivo, adrenochrome is synthesized by the oxidation of epinephrine. In vitro, silver oxide (Ag2O) is used as an oxidizing agent.[1] Its presence is detected in solution by a pink color. The color turns brown upon polymerization.
Effect on the brain
Several small-scale studies (involving 15 or fewer test subjects) conducted in the 1950s and 1960s reported that adrenochrome triggered psychotic reactions such as thought disorder, derealization, and euphoria.[2] Researchers Abram Hoffer and Humphry Osmond claimed that adrenochrome is a neurotoxic, psychotomimetic substance and may play a role in schizophrenia and other mental illnesses.[3] In what they called the "adrenochrome hypothesis",[4] they speculated that megadoses of vitamin C and niacin could cure schizophrenia by reducing brain adrenochrome.[5][6] However, these hypotheses have never been scientifically accepted; adrenochrome is not currently believed to have any psychedelic properties.[7]
Law
Adrenochrome is unscheduled by the Controlled Substances Act in the United States, but if sold as a supplement, sales must conform to U.S. supplement laws. If sold for consumption as a food or drug, sales are regulated by the FDA.[8]
In popular culture
- Adrenochrome is mentioned in The Doors of Perception by Aldous Huxley as "a product of the decomposition of adrenaline" that can "produce many of the symptoms observed in mescaline intoxication".
- Author Hunter S. Thompson mentions adrenochrome in his book Fear and Loathing in Las Vegas. The adrenochrome scene also appears in the novel's film adaptation. In the DVD commentary, director Terry Gilliam admits that his and Thompson's portrayal is a fictional exaggeration. In fact, Gilliam insists that the drug is entirely fictional and seems unaware of the existence of a substance with even a similar name. Thompson also mentions the substance in his book Fear and Loathing on the Campaign Trail '72.
- The harvesting of an adrenal gland from a live victim to obtain Adrenochrome for drug abuse is a plot feature in the 2nd episode "Whom the Gods would Destroy" of Series 1 of the British TV series Lewis (2008).[9]
- Appetite for Adrenochrome is the title of the debut album by Sacramento, California pop-punk band the Groovie Ghoulies.
- Adrenochrome is a song recorded in 1982 by The Sisters of Mercy, released on the Body Electric single and compilations including Some Girls Wander by Mistake.
- Adrenochrome is a song produced in 2005 by the electronic music producer Nhar.
- "Drencrom" is one of several substances mentioned in the beginning of A Clockwork Orange by Anthony Burgess.
References
- ↑ MacCarthy, Chim, Ind. Paris 55,435(1946)
- ↑ John Smythies (2002). "The adrenochrome hypothesis of schizophrenia revisited". Neurotoxicity Research. 4 (2): 147–150. doi:10.1080/10298420290015827.
- ↑ Hoffer, A. Osmond, H., Smithies, J.; Schizophrenia: a new approach. Journal of Mental Science #100 (January, 1954)
- ↑ Hoffer, A (1990). "The Adrenochrome Hypothesis and Psychiatry". Retrieved 2011-07-25.
- ↑ Hoffer, A. and Osmond, H. The Hallucinogens (Academic Press, 1967).
- ↑ Hoffer, A., Osmond, H., & Smythies, J. (1994). An Evolutionary Defense Against Severe Stress. Schizophrenia: A New Approach (pp. 205–221). Victoria, Canada: Journal of Orthomolecular Medicine
- ↑ "The controversy that these reports created just sort of died away, and the adrenochrome family has never been accepted as being psychedelic. No one in the scientific community today is looking in and about the area, and at present this is considered as an interesting historical footnote." As seen at: Alexander Shulgin and Ann Shulgin (1991). "#157 (TMA)". PiHKAL - A Chemical Love Story. Transform Press.
- ↑ Erowid. "Adrenochrome Law". Retrieved 2013-01-14.
- ↑ "Inspector Lewis Series Synopsis". Archived from the original on 2008-06-26.
External links
- Adrenochrome Commentary at erowid.org
- Adrenochrome deposits resulting from the use of epinephrine-containing eye drops used to treat glaucoma from the Iowa Eye Atlas (searched for diagnosis = adrenochrome)