Sea anemone
| Sea anemones | |
|---|---|
 | |
| A selection of sea anemones, painted by Giacomo Merculiano, 1893 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Cnidaria |
| Class: | Anthozoa |
| Subclass: | Hexacorallia |
| Order: | Actiniaria |
| Suborders | |
| Diversity | |
| 46 families | |
Sea anemones are a group of water-dwelling, predatory animals of the order Actiniaria. They are named for the anemone, a terrestrial flower. Sea anemones are classified in the phylum Cnidaria , class Anthozoa, subclass Hexacorallia.[1] Anthozoa often have large polyps that allow for digestion of larger prey and also lack a medusa stage.[2] As cnidarians, sea anemones are related to corals, jellyfish, tube-dwelling anemones, and Hydra.
Many sea anemones form symbiotic relationships with single-celled dinoflagellates, zooxanthellae or with green algae, zoochlorellae, that live within their cells.
The global trade in marine ornamentals for aquariums is rapidly expanding, and threatens sea anemone populations as the trade depends on collection from the wild, and the animals grow and reproduce relatively slowly.
Anatomy
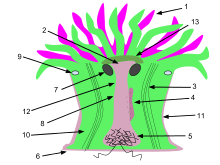
1. Tentacles 2. Mouth 3. Retracting muscles 4. Gonads 5. Acontial filaments 6. Pedal disk 7. Ostium 8. Coelenteron 9. Sphincter muscle 10. Mesentery 11. Column 12. Pharynx

A typical sea anemone is a sessile polyp attached at the base to the surface beneath it by an adhesive foot, called a basal or pedal disc, with a column-shaped body topped by an oral disc. Most are from 1 to 5 cm (0.4 to 2.0 in) in diameter and 1.5 to 10 cm (0.6 to 3.9 in) in length, but they are inflatable and vary greatly in dimensions. Some are very large; Urticina columbiana and Stichodactyla mertensii can both exceed a metre in diameter and Metridium farcimen a metre in length.[3] Some species burrow in soft sediment and lack a basal disc, having instead a bulbous lower end, the physa, which anchors them in place.[3] A few species such as Actinecta are pelagic and are not attached to the bottom; instead, they have a gas chamber within the pedal disc, allowing them to float upside down near the surface of the water.[4]
The column or trunk is generally more or less cylindrical and may be plain and smooth or may bear specialist structures; these include solid papillae (fleshy protuberances), adhesive papillae, cinclides (slits) and small protruding vesicles. In some species the part immediately below the oral disc is constricted and is known as the capitulum. When the animal contracts, the oral disc, tentacles and capitulum fold inside the pharynx and are held in place by a strong sphincter muscle part way up the column. There may be a fold in the body wall, known as a parapet, at this point, and this parapet covers and protects the anemone when it is retracted.[3]
The oral disc has a central mouth, usually slit-shaped, surrounded by one or more whorls of tentacles. The ends of the slit lead to grooves in the wall of the pharynx known as siphonoglyphs; there are usually two of these grooves, but some groups have a single one. The tentacles are generally tapered and often tipped by a pore, but in some species they are branched, club-tipped, or reduced to low knobs.[3] The tentacles are armed with many cnidocytes, cells that are both defensive and used to capture prey. Cnidocytes contain stinging nematocysts, capsule-like organelles capable of everting suddenly, giving the phylum Cnidaria its name.[5] Each nematocyst contains a small venom vesicle filled with actinotoxins, an inner filament, and an external sensory hair. A touch to the hair mechanically triggers a cell explosion, which launches a harpoon-like structure that attaches to the organism that triggered it, and injects a dose of venom in the flesh of the aggressor or prey.[6] At the base of the tentacles in some species lie acrorhagi, elongated inflatable tentacle-like organs armed with cnidocytes, that can flail around and fend off other encroaching anemones; one or both anemones can be driven off or suffer injury in such battles.[3]
The venom is a mix of toxins, including neurotoxins, that paralyzes the prey so the anemone can move it to the mouth for digestion inside the gastrovascular cavity. Actinotoxins are highly toxic to prey species of fish and crustaceans. However, Amphiprioninae (clownfish), small banded fish in various colours, are not affected by their host anemone's sting and shelter themselves from predators among its tentacles.[7] Several other species have similar adaptions and are also unaffected (see Symbiotic relationships). Most sea anemones are harmless to humans, but a few highly toxic species (notably Actinodendron arboreum, Phyllodiscus semoni and Stichodactyla ssp.) have caused severe injuries and are potentially lethal.[8]
Digestive system
A gastrovascular cavity functions as a stomach and possesses a single opening to the outside, which operates as both a mouth and anus. Waste and undigested matter is excreted through this opening, which can be described as an incomplete gut. The mouth is typically slit-like in shape, and bears a groove at one or both ends. The groove, termed a siphonophore, is ciliated, and helps to circulate water through the gastrovascular cavity.[4]
The mouth opens into a flattened pharynx. This consists of an in-folding of the body wall, and is therefore lined by the animal's epidermis. The pharynx typically runs for about one third the length of the body before opening into the gastrovascular cavity that fills the remainder of the body.
The gastrovascular cavity itself is divided into a number of chambers by mesenteries radiating inwards from the body wall. Some of the mesenteries form complete partitions with a free edge at the base of the pharynx, where they connect, but others reach only partway across. The mesenteries are usually found in multiples of twelve, and are symmetrically arranged around the central pharynx. They have stomach lining on both sides, separated by a thin layer of mesoglea, and include filaments of tissue specialised for secreting digestive enzymes. In some species, these filaments extend below the lower margin of the mesentery, hanging free in the gastrovascular cavity as thread-like acontial filaments. These acontia are armed with nematocysts and can be extruded through cinclides, blister-like holes in the wall of the column, for use in defence.[4]
Nerve system

A primitive nervous system, without centralization, coordinates the processes involved in maintaining homeostasis, as well as biochemical and physical responses to various stimuli. No specialized sense organs are present.
The muscles and nerves are much simpler than those of most other animals, although more specialised than in other cnidarians, such as corals. Cells in the outer layer (epidermis) and the inner layer (gastrodermis) have microfilaments that group into contractile fibers. These fibers are not true muscles because they are not freely suspended in the body cavity as they are in more developed animals. Longitudinal fibres are found in the tentacles and oral disc, and also within the mesenteries, where they can contract the whole length of the body. Circular fibers are found in the body wall and, in some species, around the oral disc, allowing the animal to retract its tentacles into a protective sphincter.[4]
Since the anemone lacks a skeleton, the contractile cells pull against the gastrovascular cavity, which acts as a hydrostatic skeleton. The anemone stabilizes itself by shutting its mouth, which keeps the gastrovascular cavity at a constant volume, making it more rigid. Although generally sessile, sea anemones are capable of slow movements using their pedal disc, or of swimming, using either their tentacles or by flexing their bodies.
Lifecycle

Unlike other cnidarians, anemones (and other anthozoans) entirely lack the free-swimming medusal stage of their lifecycle; the polyp produces eggs and sperm, and the fertilized egg develops into a planula that develops directly into another polyp.
The sexes in sea anemones are separate in some species, while other species are protandric hermaphrodites. The brooding anemone (Epiactis prolifera) is gynodioecious, starting life as a female and later becoming hermaphroditic, so that populations consist of females and hermaphrodites, and all females are fertilized by hermaphrodites. The gonads are strips of tissue within the mesenteries. Both sexual and asexual reproduction can occur. In sexual reproduction, males release sperm to stimulate females to release eggs, and fertilization occurs. Anemones eject eggs and sperm through the mouth. The fertilized egg develops into a planula, which settles and grows into a single polyp.
Anemones can also reproduce asexually, by budding or in some cases by binary fission, when the polyp separates into two halves. Some species can also reproduce by pedal laceration. In this process, a ring of material breaks off from the pedal disc at the base of the column which then fragments, the pieces regenerating into new individuals.[9] The sea anemone Aiptasia diaphana displays sexual plasticity. Thus asexually produced clones derived form a single founder individual can contain both male and female individuals (ramets).[10] When eggs and sperm (gametes) are formed, they can produce zygotes derived from “selfing” (within the founding clone) or out-crossing, that then develop into swimming planula larvae.
Behaviour and ecology

The sea anemone has an oral disk, which the organism uses to capture prey. The anemone attaches to the substrate using the basal disk at its posterior end. Others also burrow into weaker objects. Some species attach to kelp while others are free-swimming.
Movement
A sea anemone is capable of changing its shape dramatically. The column and tentacles have longitudinal, transverse and diagonal sheets of muscle and can lengthen and contract, as well as bend and twist. The gullet and mesenteries can evert (turn inside out), or the oral disc and tentacles can retract inside the gullet, with the sphincter closing the aperture; during this process, the gullet folds transversely and water is discharged through the mouth.[11]
Locomotion
Although a few species of sea anemone are pelagic and others burrow in soft sediment, the majority attach to a hard surface with their pedal disc and tend to stay in the same spot for weeks or months at a time. They can move however, being able to creep around on their bases; this gliding can be seen with time-lapse photography but the motion is so slow as to be almost imperceptible to the naked eye.[12] The process resembles the locomotion of a gastropod mollusc, a wave of contraction moving from the functionally posterior portion of the foot towards the front edge, which detaches and moves forwards.[13] Sea anemones can also cast themselves loose from the substrate and drift to a new location.[12] Gonactinia prolifera is unusual in that it can both walk and swim; walking is by making a series of short, looping steps, rather like a caterpillar, attaching its tentacles to the substrate and drawing its base closer; swimming is done by rapid movements of the tentacles beating synchronously like oar strokes.[14]
Although most sea anemones attach temporarily to submerged objects, a few thrust themselves into the sand or live in burrows. The sea onion Paranthus rapiformis lives on subtidal mud flats and burrows into the sediment, holding itself in place by expanding its basal disc to form an anchor. If it gets washed out of its burrow by strong currents, it contracts into a pearly glistening ball.[15] Tube-dwelling anemones which live in parchment-like tubes, are in the anthozoan subclass Ceriantharia, and are only distantly related to sea anemones.[16]
Feeding and diet
Sea anemones are typically predators, ensnaring prey of suitable size that comes within reach of their tentacles and immobilizing it with the aid of their nematocysts.[17]. The prey is then transported to the mouth and thrust into the pharynx. The lips can stretch to aid in prey capture and can accommodate larger items such as crabs, dislodged molluscs and even small fish.[3]
Some anemones feed on small particles, which are caught with the aid of a mucus secretion and moving currents that are set up by the tentacles. These usually have large numbers of tentacles and include the plumose anemones and members of the Stichodactylidae family; Stichodactyla helianthus however is reported to trap sea urchins by enfolding them in its carpet-like oral disc.[3] A few species are parasitic on other marine organisms.[17] One of these is Peachia quinquecapitata, the larvae of which develop inside the medusae of jellyfish, feeding on their gonads and other tissues, before being liberated into the sea as free-living, juvenile anemones.[18]
Although not plants and therefore incapable of photosynthesis themselves, many sea anemones form an important facultative symbiotic relationship with certain single-celled algae species that reside in the animals' gastrodermal cells, especially in the tentacles and oral disc. These algae may be either zooxanthellae, zoochlorellae or both. The sea anemone benefits from the products of the algae's photosynthesis, namely oxygen and food in the form of glycerol, glucose and alanine; the algae in turn are assured a reliable exposure to sunlight and protection from micro-feeders, which the sea anemones actively maintain. The algae also benefit by being protected by the sea anemone's stinging cells, nematocysts, reducing the likelihood of being eaten by herbivores. In the aggregating anemone (Anthopleura elegantissima), the colour is largely dependent on the proportions and identities of the zooxanthellae and zoochlorellae present.[3] the hidden anemone (Lebrunia coralligens) has a whorl of seaweed-like pseudotentacles, rich in zooxanthellae, and an inner whorl of tentacles. A daily rhythm sees the pseudotentacles spread widely in the daytime for photosynthesis, but they are retracted at night, at which time the tentacles expand to search for prey.[19]
Symbiotic relationships
Several species of fish and invertebrates live in symbiotic or commensal relationships with sea anemones, most famously the clownfish. The symbiont receives the protection from predators provided by the anemone's stinging cells, and the anemone utilises the nutrients present in its faeces.[20] Other animals that associate with sea anemones include cardinalfish (such as Banggai cardinalfish), juvenile threespot dascyllus,[21] Bucchich's (or anemone) goby,[22] juvenile painted greenling,[23] various crabs (such as Inachus phalangium, Mithraculus cinctimanus and Neopetrolisthes), shrimp (such as certain Alpheus, Lebbeus, Periclimenes and Thor),[24] opossum shrimp (such as Heteromysis and Leptomysis),[25] and various marine snails.[26][27][28]
Two of the more unusual relationships are those between certain anemones (such as Adamsia, Calliactis and Neoaiptasia) and hermit crabs or snails, and Bundeopsis or Triactis anemones and Lybia boxing crabs. In the former, the anemones live on the shell of the hermit crab or snail.[24][26][27][28] In the latter, the small anemones are carried in the claws of the boxing crab.[24][29]
Habitats
Most species inhabit tropical reefs, although there are species adapted to relatively cold waters, intertidal reefs, and sand/kelp environments.
Exploitation

The global trade of marine ornamentals has been a rapidly expanding industry involving numerous countries worldwide. In the early 1980s, the estimated value of imported marine fish and invertebrates was US$24–40 million annually.[30] Current estimates place that value at US$200–330 million,[31] with the United States accounting for 80% of the industry imports.[32] Despite advances and the expansion of aquaculture, postlarval capture and rearing, the majority of marine ornamentals are collected in the wild as adults or juveniles.[33]
Anemones are susceptible to overexploitation due to their long lifespans, slower relative growth rates, and lower reproductive rates than their resident fish, which are also affected because they settle exclusively and are restricted to specific host sea anemones. The demand for these organisms is reflected in fishermen's catch records, which document the value they are paid per catch, and on average sea anemones were valued at five times the average value of anemonefish, and 10 times the value of the most abundant anemonefish, and in fact only made up 4.1% of the total value of the catch.
Aquarium fishing activities significantly impact the populations of anemones and anemonefish by drastically reducing the densities of each in exploited areas,[33] and could also negatively affect anemone shrimp, and any organisms obligately associated with sea anemones. Anemonefish can survive alone in captivity, as has been shown by multiple research efforts.[34][35]
In southern Italy and southwestern Spain, the anemone Anemonia sulcata is consumed as a delicacy.[36] The whole animal is marinated in vinegar, then coated in a batter similar to that of fried calamari and deep-fried in olive oil.
Fossil record
Most Actiniaria do not form hard parts that can be recognized as fossils, but a few do exist; Mackenzia, from the Middle Cambrian Burgess Shale of Canada, is the oldest fossil identified as a sea anemone.
Taxonomy
Rodriguez et al. proposed a new classification for the Actiniaria based on extensive DNA results.[37]
Suborders and Superfamilies included in Actiniaria are:
- Suborder Anenthemonae
- Superfamily Edwardsioidea
- Superfamily Actinernoidea
- Suborder Enthemonae
- Superfamily Actinostoloidea
- Superfamily Actinioidea
- Superfamily Metridioidea
The relationships of higher-level taxa in Carlgren’s[38] classification are re-interpreted as follows:
| Carlgren taxon | Phylogenetic result |
|---|---|
| Protantheae | Sister to Boloceroidaria |
| Ptychodacteae | Polyphyletic because its members are not recovered as sister taxa; clustered with members of former Endomyaria |
| Endocoelantheae | Sister to athenarian family Edwardsiidae; together these clades are re-classified as suborder Anenthemonae |
| Nynantheae | Polyphyletic because of the relationship between Edwardsiidae and Endocoelantheae and because members of Protantheae and Ptychodacteae are recovered as sister to its members |
| Boloceroidaria | Boloceroides mcmurrichi and Bunodeopsis nested among acontiate taxa; B. daphneae apart from other Actiniaria |
| Athenaria | Polyphyletic: families formerly in this suborder distributed across tree as sister to former members of Endomyaria, Acontiaria, and Endocoelantheae |
| Thenaria | Boloceroidaria, Protantheae, Ptychodacteae, and most Athenaria nest within this group |
| Endomyaria | Paraphyletic: includes Pychodacteae and some Athenaria |
| Mesomyaria | Polyphyletic: one clade at base of Nynantheae, other lineages are associated with former members of Acontiaria |
| Acontiaria | Paraphyletic; includes several lineages formerly in Mesomyaria and Athenaria, plus Boloceroidaria and Protantheae |
See also
References
- ↑ Daly, Marymegan; Brugler, Mercer R.; Cartwright, Paulyn; Collins, Allen G.; Dawson, Michael N.; Fautin, Daphne G.; France, Scott C.; McFadden, Catherine S.; Opresko, Dennis M.; Rodriguez, Estefania; Romano, Sandra L.; Stake, Joel L. (2007). "The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus" (PDF). Zootaxa. 1668: 127–182. ISSN 1175-5334.
- ↑ Castro, Peter; Huber, Michael E. (2010). Marine Biology. New York: McGraw-Hill. p. 121.
- 1 2 3 4 5 6 7 8 Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 130–132. ISBN 978-81-315-0104-7.
- 1 2 3 4 Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 150–157. ISBN 0-03-056747-5.
- ↑ Campbell, N.; Reece, J. (2002). Biology (6th ed.). San Francisco: Pearson Education. ISBN 978-0-8053-0009-3.
- ↑ Beckmann, A.; Özbek, S. (2012). "The nematocyst: a molecular map of the cnidarian stinging organelle". International Journal of Developmental Biology. 56 (6–8): 577–582. PMID 22689365. doi:10.1387/ijdb.113472ab.
- ↑ "Fagatele Bay NMS: Clownfish and Sea Anemone". National Marine Sanctuaries. January 12, 2006. Retrieved 2010-11-20.
- ↑ Mizuno, Masashi; Ito, Yasuhiko; Morgan, B. Paul (23 July 2012). "Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat". Marine Drugs. 10 (7): 1582–1604. doi:10.3390/md10071582.
- ↑ Smith, Nathan III; Lenhoff, Howard M. (1976). "Regulation of Frequency of Pedal Laceration in a Sea Anemone". Coelenterate Ecology and Behavior: 117–125. doi:10.1007/978-1-4757-9724-4_13.
- ↑ Schlesinger A; Kramarsky-Winter E; Rosenfeld H; Armoza-Zvoloni R; Loya Y (2010). "Sexual plasticity and self-fertilization in the sea anemone Aiptasia diaphana". PLoS ONE. 5 (7): e11874. PMC 2912375
 . PMID 20686700. doi:10.1371/journal.pone.0011874.
. PMID 20686700. doi:10.1371/journal.pone.0011874. - ↑ Kotpal, R.L. (2012). Modern Text Book of Zoology: Invertebrates. Rastogi Publications. pp. 286–292. ISBN 978-81-7133-903-7.
- 1 2 Horton, Andy. "Sea anemones". British Marine Life Study Society. Retrieved 2017-07-13.
- ↑ McClendon, J.F. (January 1906). "On the locomotion of a sea anemone (Metridium marginatum)". doi:10.2307/1535667.
- ↑ Robson, Elaine A. (1971). "The Behaviour and Neuromuscular System of Gonactinia prolifera, A Swimming Sea-Anemone". Journal of Experimental Biology. 55: 611–640.
- ↑ Lippson, Robert L.; Lippson, Alice Jane (2009). Life along the Inner Coast: A Naturalist's Guide to the Sounds, Inlets, Rivers, and Intracoastal Waterway from Norfolk to Key West. University of North Carolina Press. p. 181. ISBN 978-0-8078-9859-8.
- ↑ Molodtsova, T. (2015). Cerianthidae. In: Fautin, Daphne G. (2011) Hexacorallians of the World. Accessed through: World Register of Marine Species on 13 July 2017
- 1 2 "Sea anemone". Columbia Encyclopedia (6th ed.). 2008.
- ↑ Spaulding, James G. (1972). "The life cycle of Peachia quinquecapitata, an anemone parasitic on medusae during its larval development" (PDF). The Biological Bulletin. 143 (2): 440–52. doi:10.2307/1540065.
- ↑ Lewis, John B. (1984). "Photosynthetic production by the coral reef anemone, Lebrunia coralligens Wilson, and behavioral correlates of two nutritional strategies". Biological Bulletin. 167 (3): 601–612. doi:10.2307/1541413.
- ↑ Jakimovsk, Bojani (2011). Biologically Inspired Approaches for Locomotion, Anomaly Detection and Reconfiguration for Walking Robots. Springer Science & Business Media. p. 69. ISBN 978-3-642-22505-5.
- ↑ Lieske, E.; Myers, R. (1999). Coral Reef Fishes. ISBN 0-691-00481-1
- ↑ Debelius, H. (1997). Mediterranean and Atlantic Fish Guide. ISBN 978-3925919541
- ↑ Fretwell, K.; and B. Starzomski (2014). Painted greenling. Biodiversity of the Central Coast. Retrieved 2015-01-29.
- 1 2 3 Debelius, H. (2001). Crustacea Guide of the World. ISBN 978-3931702748
- ↑ Wittmann, K.J. (2008). Two new species of Heteromysini (Mysida, Mysidae) from the island of Madeira (N.E. Atlantic), with notes on sea anemone and hermit crab commensalisms in the genus Heteromysis S. I. Smith, 1873. Crustaceana, 81(3): 351–374.
- 1 2 Mercier, A.; and J. Hamel (2008). Nature and role of newly described symbiotic associations between a sea anemone and gastropods at bathyal depths in the NW Atlantic. Journal of Experimental Marine Biology and Ecology 358(1): 57-69.
- 1 2 Goodwill, R.H.; D.G. Fautin; J. Furey; and M. Daly (2009). A sea anemone symbiotic with gastropods of eight species in the Mariana Islands. Micronesica 41(1): 117–130.
- 1 2 Ates, R.M.L. (1997). Gastropod carrying actinians. In: J. C. den Hartog, eds, Proceedings of the 6th International Conference on Coelenterate Biology, 1995, pp. 11-20. National Naturhistorisch Museum, Leiden, The Netherlands.
- ↑ Karplus, I; G.C. Fiedler; and P. Ramcharan (1998). The intraspecific fighting behavior of the Hawaiian Boxing Crab, Lybia edmondsoni - Fighting with dangerous weapons? Symbiosis 24: 287-302.
- ↑ Eliabeth M. Wood (1985). Exploitation of Coral Reef Fishes for the Aquarium Trade. Report to the Marine Conservation Society.
- ↑ Frank A. Chapman; Sharon A. Fitz-Coy; Eric M. Thunberg; Charles M. Adams (March 1997). "United States of America trade in ornamental fish" (PDF). Journal of the World Aquaculture Society. 28 (1): 1–10. doi:10.1111/j.1749-7345.1997.tb00955.x.
- ↑ Sherry L. Larkin & Robert L. Degner (2001). "The U.S. wholesale market for marine ornamentals". Aquarium Sciences and Conservation. 3 (1–3): 13–24. doi:10.1023/A:1011329300967.
- 1 2 Craig S. Shuman, Gregor Hodgson & Richard F. Ambrose (December 2005). "Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines" (PDF). Coral Reefs. 24 (4): 564–573. doi:10.1007/s00338-005-0027-z.
- ↑ Daphne Gail Fautin (1991). "The anemonefish symbiosis: what is known and what is not" (PDF). Symbiosis. 10: 23–46. Archived from the original (PDF) on 2012-05-25.
- ↑ Ronald L. Shimek (2004). Marine Invertebrates. Neptune City, NJ: T.F.H. Publications. p. 83. ISBN 978-1-890087-66-1.
- ↑ Schwabe, Calvin W. (1979). Unmentionable cuisine. Charlottesville: University Press of Virginia. p. 362. ISBN 0-8139-0811-6.
- ↑ Rodríguez, E., Barbeitos, M. S., Brugler, M.R., Crowley, L. M., Grajales, A., Gusmão, L., Häussermann, V., Reft, A. & Daly, M. (2104). Hidden among sea anemones: The first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS ONE 9(5): e96998. doi:10.1371/journal.pone.0096998
- ↑ Carlgren O. (1949). A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. K Svenska VetenskapsAkad Handl 1: 1–121.
External links
| Wikispecies has information related to: Actiniaria |
| Wikimedia Commons has media related to Actiniaria. |
| Look up Actiniaria in Wiktionary, the free dictionary. |
- Order Actiniaria
- Actiniaria.com
- Photos of various species of Sea Anemones from the Indopacific
- Anemone Armies Battle to a Standoff
- Anemone Wars: Clone armies deploy scouts, attack tidally – unsuspected military tactics
- Sea anemones look like sea flowers but they are animals of the Phylum Cnidaria
- Information about Ricordea Florida Sea anemones & pictures
- Photographic Database of Cambodian Sea Anemones
- Photos of Sea Anemones