Acetogenin
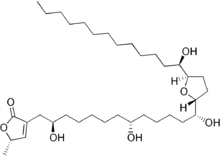
Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahydrofurans and tetrahydropyrans. They are often terminated with a lactone or butenolide.[1] Over 400 members of this family of compounds have been isolated from 51 different species of plants.[2]
Examples include:
Structure
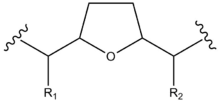
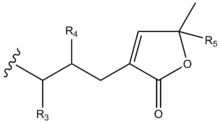
Structurally, acetogenins are a series of C-35/C-37 compounds usually characterized by a long aliphatic chain bearing a terminal methyl-substituted α,β-unsaturated γ-lactone ring, as well as one to three tetrahydrofuran (THF) rings.[3] These THF rings are located along the hydrocarbon chain, along with a number of oxygenated moieties (hydroxyls, acetoxyls, ketones, epoxides) and/or double bonds.[3]
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 4-deoxyannoreticuin | OH | OH | H | H | H |
| Annonacin | OH | OH | H | OH | H |
| Annopentocin A | OH | H | H | OH | H |
| Dispalin | OAc | OH | H | OH | H |
| Donnaienin C | OH | OH | H | OAc | OH |
| Goniotetracin | OH | OH | H | OH | H |
| Muricoreacin | OH | H | H | OH | H |
| Tonkinin A | OH | OH | O | H | H |
| Uvaribonone | OH | OAc | O | H | H |
Biological effects
Acetogenins have been investigated for their potential therapeutic use in treating cancer,[4] but this potential is tempered with concerns about neurotoxicity.[5][6][7][8][9]
Purified acetogenins and crude extracts of the common North American pawpaw (Asimina triloba) or the Brazilian pawpaw (Annona muricata) remain under basic research for their possible biological properties.[10][11]
References
- ↑ Li, N.; Shi, Z.; Tang, Y.; Chen, J.; Li, X. (2008). "Recent Progress on the Total Synthesis of Acetogenins from Annonaceae" (PDF). Beilstein Journal of Organic Chemistry. 4 (48): 1–62. PMC 2633664
 . PMID 19190742. doi:10.3762/bjoc.4.48.
. PMID 19190742. doi:10.3762/bjoc.4.48. - ↑ Bermejo, A.; Figadère, B.; Zafra-Polo, M.-C.; Barrachina, I.; Estornell, E.; Cortes, D. (2005). "Acetogenins from Annonaceae: Recent Progress in Isolation, Synthesis and Mechanisms of Action". Natural Product Reports. 22 (2): 269–303. PMID 15806200. doi:10.1039/B500186M. Erratum: "Back Matter". Natural Product Reports. 22 (3): 426. 2005. doi:10.1039/B503508M.
- 1 2 3 Alali, F. Q., Liu, X., & Mclaughlin, J. L. (1999). Annonaceous Acetogenins: Recent Progress. Journal of Natural Products, 62(3), 504-540. doi:10.1021/np980406d
- ↑ Mangal, M; Khan, M. I.; Agarwal, S. M. (2015). "Acetogenins as Potential Anticancer Agents". Anti-cancer agents in medicinal chemistry. 16 (2): 138–59. PMID 26118710. doi:10.2174/1871520615666150629101827.
- ↑ Ana V. Coria-Téllez, Efigenia Montalvo-Gónzalez, Elhadi M. Yahia, Eva N. Obledo-Vázquez (2016). "Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity". Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2016.01.004.
- ↑ Le Ven, J.; Schmitz-Afonso, I.; Touboul, D.; Buisson, D.; Akagah, B.; Cresteil, T.; Lewin, G.; Champy, P. (2011). "Annonaceae fruits and parkinsonism risk: Metabolisation study of annonacin, a model neurotoxin; evaluation of human exposure". Toxicology Letters. 205: S50. doi:10.1016/j.toxlet.2011.05.197.
- ↑ Victor R. Preedy; Ronald Ross Watson; Vinood B. Patel, eds. (2011). Nuts and Seeds in Health and Disease Prevention. Academic Press. pp. 434–435.
- ↑ Robert A. Levine, Kristy M. Richards, Kevin Tran, Rensheng Luo, Andrew L. Thomas, and Robert E. Smith (2015). "Determination of Neurotoxic Acetogenins in Pawpaw (Asimina triloba) Fruit by LC-HRMS". J. Agric. Food Chem. 63 (4): 1053–1056. PMID 25594104. doi:10.1021/jf504500g.
- ↑ Potts, L. F.; Luzzio, F. A.; Smith, S. C.; Hetman, M; Champy, P; Litvan, I (2012). "Annonacin in Asimina triloba fruit: Implication for neurotoxicity". Neurotoxicology. 33 (1): 53–8. PMID 22130466. doi:10.1016/j.neuro.2011.10.009.
- ↑ McLaughlin, J.L. (2008). "Paw paw and cancer: annonaceous acetogenins from discovery to commercial products.". Journal of Natural Products. 71 (7): 1311–21. PMID 18598079. doi:10.1021/np800191t.
- ↑ Coothankandaswamy, Veena; Liu, Yang; Mao, Shui-Chun; Morgan, J. Brian; Mahdi, Fakhri; Jekabsons, Mika B.; Nagle, Dale G.; Zhou, Yu-Dong (2010). "The Alternative Medicine Pawpaw and Its Acetogenin Constituents Suppress Tumor Angiogenesis via the HIF-1/VEGF Pathway". Journal of Natural Products. 73 (5): 956–961. PMC 2890309
 . PMID 20423107. doi:10.1021/np100228d.
. PMID 20423107. doi:10.1021/np100228d.
External links
-
 Media related to Acetogenins at Wikimedia Commons
Media related to Acetogenins at Wikimedia Commons