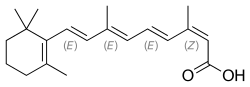
Isotretinoin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | See note at tretinoin |
| Trade names | Accutane (originator), subsequently many generics[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681043 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Variable |
| Protein binding | 99.9% |
| Metabolism | Hepatic |
| Biological half-life | 10–20 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.022.996 |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.44 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Isotretinoin, also known as 13-cis-retinoic acid, is an medication primarily used to treat severe acne. Rarely, it is also used to prevent certain skin cancers (squamous-cell carcinoma), and in the treatment of other cancers. It is used to treat harlequin-type ichthyosis, a usually lethal skin disease, and lamellar ichthyosis. It is a retinoid, meaning it is related to vitamin A, and is found in small quantities naturally in the body. Its isomer, tretinoin, is also an acne drug.
Isotretinoin is primarily used as a treatment for severe acne. The most common adverse effects are a transient worsening of acne (lasting 2–3 weeks), dry lips (cheilitis), dry and fragile skin, and an increased susceptibility to sunburn. Uncommon and rare side effects include muscle aches and pains (myalgias), and headaches. Isotretinoin is known to cause birth defects due to in utero exposure because of the molecule's close resemblance to retinoic acid, a natural vitamin A derivative which controls normal embryonic development. It is also associated with psychiatric side effects, including depression.
In the United States, a special procedure is required to obtain the pharmaceutical. In most other countries, a consent form is required which explains these risks. Women taking isotretinoin must not get pregnant during and for 1 month after the discontinuation of isotretinoin therapy. Sexual abstinence or effective contraception is mandatory during this period. Barrier methods by themselves (e.g., condoms) are not considered adequate due to the unacceptable failure rates of approximately 3%. Women who fall pregnant whilst on isotretinoin therapy are generally counselled to have a termination. Isotretinoin has no effect on male fertility.[2]
Isotretinoin was first marketed as Accutane by Hoffmann-La Roche. It sold well for many years, but in 2009, Roche decided to remove Accutane from the US market after juries had awarded millions of dollars in damages to former Accutane users over inflammatory bowel disease claims. It then became generic and as of 2017 was marketed under many brand names worldwide.[1]
Medical uses
Isotretinoin is used primarily for severe cystic acne and acne that has not responded to other treatments.[3][4][5][6] Many dermatologists also support its use for treatment of lesser degrees of acne that prove resistant to other treatments, or that produce physical or psychological scarring.[7]
It is also somewhat effective for hidradenitis suppurativa and some cases of severe acne rosacea.[8] It can also be used to help treat harlequin ichthyosis, lamellar ichthyosis and is used in xeroderma pigmentosum cases to relieve keratoses. Isotretinoin has been used to treat the extremely rare condition fibrodysplasia ossificans progressiva. It is also used for treatment of neuroblastoma, a form of nerve cancer.
Isotretinoin therapy has furthermore proven effective against genital warts in experimental use, but is rarely used for this indication as there are more effective treatments. Isotretinoin may represent an efficacious and safe alternative systemic form of therapy for recalcitrant condylomata acuminata (RCA) of the cervix. In most countries this therapy is currently unapproved and only used if other therapies failed.[9][10]
Prescribing restrictions
Isotretinoin is a teratogen; there is about a 20–35% risk for congenital defects in infants exposed to the drug in utero, and about 30–60% of children exposed to isotretinoin prenatally have been reported to show neurocognitive impairment.[11] Because of this, there are strict controls on prescribing isotretinoin to women who may become pregnant and women who become pregnant while taking isotretinoin are strongly advised to terminate their pregnancies.[11]
In most countries, isotretinoin can only be prescribed by dermatologists or specialist physicians; some countries also allow limited prescription by general practitioners and family doctors. In the United Kingdom[12] and Australia,[13][14] isotretinoin may be prescribed only by or under the supervision of a consultant dermatologist. Because severe cystic acne has the potential to cause permanent scarring over a short period, restrictions on its more immediate availability have proved contentious.[15] In New Zealand, isotretinoin can be prescribed by any doctor but subsidised only when prescribed by a vocationally-registered general practitioner, dermatologist or nurse practitioner.[16]
In the United States, dispensing of isotretinoin is by an FDA-mandated website called iPLEDGE. iPLEDGE has applied to isotretinoin prescriptions since 1 March 2006. Under it, dermatologists must register their patients on the system before prescribing isotretinoin. Pharmacists must then verify the prescription on the iPLEDGE website before dispensing isotretinoin. The website allows no more than thirty days' supply of the drug to be prescribed or dispensed; and after issuance, another prescription may not be written for at least 30 days (even in the case of lost prescriptions). Prescriptions expire from iPLEDGE if not picked up from the pharmacy seven days after issuance. Physicians and pharmacists must verify written prescriptions on the system before filling an isotretinoin prescription. Due to the teratogenic effects of isotretinoin, iPLEDGE makes additional requirements of female patients filling prescriptions for the drug: women with child-bearing potential must commit to using two forms of effective contraception simultaneously for the duration of isotretinoin therapy and for a month immediately preceding and a month immediately following therapy. Additionally they must have two negative pregnancy tests 30 days apart and have negative pregnancy tests before each prescription is written.[17] Alerts continue to exist against purchasing isotretinoin online.[18]
Adverse effects
Increasingly higher dosages will result in higher toxicity, resembling vitamin A toxicity. The following are adverse drug reactions from Roche's UK product information for Roaccutane as of October 2010:[19]
|
Type of disorders |
Very common (≥ 1/10) |
Common (≥ 1/100, < 1/10) |
Rare (≥ 1/10 000,< 1/1000) |
Very rare (≤ 1/10 000) |
|---|---|---|---|---|
| Infections |
bacterial infection | |||
| Blood and lymphatic system |
|
| ||
| Immune system |
|
|||
| Metabolism | ||||
| Psychiatric |
|
| ||
| Nervous system |
|
|||
| Eye |
|
| ||
| Ear |
| |||
| Vascular |
allergic vasculitis) | |||
| Respiratory, thoracic and |
|
with asthma) | ||
| Gastrointestinal |
| |||
| Hepatobiliary |
|
|||
| Skin and |
|
| ||
| Musculo-skeletal and |
|
and tendons)
| ||
| Kidney and urinary | ||||
| General |
| |||
| Investigation |
|
|
| |
Possible permanent effects
Isotretinoin may stop long bone growth in young people who are still growing.[6] Several reports state that premature epiphyseal closure can occur in acne patients receiving recommended doses of Accutane.[20][21][22][23]
Isotretinoin is known to cause meibomian gland dysfunction which causes persistent keratoconjunctivitis sicca (dry eye).[24] It has also been found to cause salivary gland dysfunction in some people, causing xerostomia (dry mouth).[25][26][27][28] Problems with the meibomian and salivary glands are likely due to the non-selective apoptosis of the cells of the exocrine glands.[29] Decreased night vision has been reported to persist in some patients after discontinuation of isotretinoin therapy.[30][31]
Hyperostosis has been reported in patients receiving treatment with isotretinoin.[32][33]
Skin and mucocutaneous tissue
The most common side effects are mucocutaneous: dry lips, skin and nose. Other common mucocutaneous side effects are inflammation and chapping of the lips (cheilitis), redness of the skin (erythema), rashes, peeling, eczema (dermatitis), itching (prunitis) and nose bleeds (epistaxis).[34] Absence of dryness of the lips is considered an indication of non-compliance with treatment (not taking the drug as advised), as it occurs in almost all patients.[34]
Regular use of lip balm and moisturizer is recommended throughout a course of treatment to reduce these problems. The dose may need to be decreased to reduce the severity of these side effects.[35] The skin becomes more fragile—especially to frictional forces—and may not heal as quickly as normal. For this reason waxing of hair, tattooing, tattoo removal, piercings, dermabrasion, exfoliation, etc. are not recommended. Treatment of acne scars is generally deferred until 12 months after completion of a course of isotretinoin.
Acne usually flares up 2–3 weeks into the treatment and is usually mild and tolerable. Occasionally this flare-up is severe, necessitating oral antiobiotics such as erythromycin. A short course of oral prednisolone may be required. Some dermatologists favour a few weeks pre-treatment with oral antibiotics before commencing isotretinoin to reduce the chance of a severe flare. A "stepped" course may also be used to reduce the chance of this initial flare, by which the initial dose is low (e.g. 0.5 mg/kg) and subsequently increased throughout the course.
Isotretinoin use can rarely lead to a more severe form of acne, acne fulminans.
Teratogenicity
Isotretinoin is a teratogen highly likely to cause birth defects if taken by women during pregnancy or even a short time before conception. A few of the more common birth defects this drug can cause are hearing and visual impairment, missing or malformed earlobes, facial dysmorphism, and abnormalities in brain function. Isotretinoin is classified as FDA Pregnancy Category X and ADEC Category X, and use is contraindicated in pregnancy.[8]
The manufacturer recommends pregnancy be excluded in female patients two weeks prior to commencement of isotretinoin, and they should use two simultaneous forms of effective contraception at least one month prior to commencement, during, and for at least one month following isotretinoin therapy.[36]
In the U.S., more than 2,000 women have become pregnant while taking the drug between 1982 and 2003, with most pregnancies ending in abortion or miscarriage. About 160 babies with birth defects were born. As a consequence, the iPLEDGE program was introduced by the U.S. FDA on 12 August 2005 in an attempt to ensure female patients receiving isotretinoin do not become pregnant. As of 1 March 2006, only prescribers registered and activated in iPLEDGE are able to prescribe isotretinoin, and only patients registered and qualified in iPLEDGE will be able to have isotretinoin dispensed by a registered pharmacy. All patients, including women not of child-bearing age and men, must register with iPLEDGE. FDA's intent with the iPLEDGE program is to tightly control the distribution and dispensing of isotretinoin and thereby prevent the potential for distribution or sharing of the drug outside of the program to women of child-bearing age. In 2011, 155 pregnancies occurred among 129,544 women of childbearing potential taking isotrentinoin (0.12%)[37]
Patients receiving isotretinoin therapy are not permitted to donate blood during and for at least one month after discontinuation of therapy due to its teratogenicity.[38]
Psychological effects
The association between isotretinoin use and psychopathology has been controversial. Beginning in 1983, isolated case reports emerged suggesting mood change, particularly depression, occurring during or soon after isotretinoin use.[39] A number of studies have been conducted since then of the drug's effect on depression, psychosis, suicidal thoughts and other psychological effects.[39]
The product information leaflet distributed with isotretinoin states that rare psychological side effects may include depression, worsening of pre-existing depression, aggressive tendencies and anxiety. Very rare effects include abnormal behaviour, psychosis, suicidal ideation, suicide attempts and suicide.[5] In a total of 5577 adverse reactions reported to the UK's MHRA up to 31 March 2017, the majority (1207, or 22%) concerned psychiatric effects.[40] There were 85 reports of suicidal ideation, 56 of completed suicide and 43 of suicide attempts.[40] Isotretinoin is the only non-psychiatric drug on the FDA's top 10 list of drugs associated with depression[41] and is also within the top 10 for suicide attempts.[42]
In 2012, a systematic review covering all articles in the literature related to isotretinoin, depression and suicide, as well as articles related to class effect, dose response, and biologic plausibility found that the literature reviewed was consistent with an association of isotretinoin administration and depression and with suicide in a subgroup of vulnerable individuals.[43] Following this systematic review, in a 2014 review a group of Australian dermatologists and psychiatrists collaborated on a set of recommendations for safe prescribing of isotretinoin.[44] However, whether isotretinoin use is causally associated with mental illness remains controversial.[44] In a survey published in February 2015, 37% of 591 dermatologists stated that they believe that isotretinoin may cause psychiatric disturbances.[45]
Evidence for depression being causally associated with isotretinoin use includes 41 reports of positive challenge/dechallenge/rechallenge with isotretinoin, involving administering isotretinoin, withdrawing the drug and then re-administering it.[43] The majority of these cases had no psychiatric history.[43] Bremner et al. state that "these examples of depression resulting from isotretinoin use ... make for a very strong case for their link in some individuals."[43] There is also a temporal relationship between development of depression and initiation of isotretinoin treatment, with most cases developing after 1–2 months of treatment.[43] Further, higher doses of isotretinoin increases the risk of developing depression, with 25% of patients showing depression on a dose of 3 mg/kg/day as compared with 3-4% at normal doses.[43]
Rarely, irritable mood and aggression have been reported during isotretinoin use.[43][46][41][47]
Some research studies emphasise the potential for positive behavioural outcomes of isotretinoin treatment associated with its efficacy in treating acne and subsequent improvements in self-esteem. However, such studies are unable to support the conclusion that acne results in clinical depression, nor that isotretinoin treatment leads to an improvement in clinical measures of depression and/or anxiety.[43] Many of the studies cited to support the existence of a relationship between isotretinoin treatment and decreased symptoms of depression and/or anxiety have significant methodological flaws. For instance, one of the most commonly cited studies[48] involved just 10 patients assessed with unstructured psychiatric interview, involved no control group and didn't report any statistics.[43]
Biological basis
A possible biological basis for the case reports of depression involves decreased metabolism in the orbitofrontal cortex (OFC) of the frontal lobe.[43]
Studies in mice and rats have found that retinoids, including isotretinoin, bind to dopaminergic receptors in the central nervous system.[41][49][50] Isotretinoin may affect dopaminergic neurotransmission by disrupting the structure of dopamine receptors and decreasing dopaminergic activity.[47] The dopaminergic system is implicated in numerous psychological disorders, including depression. Isotretinoin is also thought to affect the serotonergic system - it increases expression of 5-HT1A receptors in the pre-synaptic neuron, which inhibit serotonin secretion.[47] Isotretinoin also directly and indirectly increases the translation of the serotonin transporter protein, leading to increased reuptake and consequently reduced synaptic availability of serotonin.[47]
Inhibition of hippocampal neurogenesis may also play a role in the development of isotretinoin-induced depression.[43] A further effect of isotretinoin on the brain involves retinoic acid function in the hypothalamus, the hormone regulatory centre of the brain and part of the hypothalamus-pituitary-adrenal axis, a key part of the body's stress response.[43] Other brain regions regulated by retinoic acid and potentially disrupted by isotretinoin include the frontal cortex and the striatum.[43]
Musculoskeletal
Isotretinoin has a number of muscoloskeletal effects. Myalgia (muscular pain) and arthralgia (joint pain) are very common side effects.[51] Retinoids, including isotretinoin, are well-known to cause bone changes, the most common type of which is hyperostotic changes (excessive bone growth), especially in growing children and adolescents.[51] Other problems include premature epiphyseal closure and calcification of tendons and ligaments.[51] The bones of the spine and feet are most commonly affected. Risk factors for skeletal effects include older age, greater dosage and longer course of treatment. Most bone changes cause no symptoms and may only be noticed using X-ray imaging.[51]
Gastrointestinal
Isotretinoin may cause non-specific gastrointestinal symptoms including nausea, diarrhoea and abdominal pain.[34] The drug is associated with inflammatory bowel disease (IBD) - ulcerative colitis, but not Crohn's disease.[52] There are also reports of patients developing Irritable Bowel Syndrome (IBS) and worsening of existing IBS.[53]
Ocular
Isotretinoin and other retinoids are well known to affect the eyes. Dry eyes are very common during treatment and is caused by isotretinoin's apoptotic effect on the meibomian glands. Some patients develop contact lens intolerance as a result.[34] In some patients, these changes are long-lasting or irreversible and represent Meibomian Gland Dysfunction (MGD).[24] Other common effects on the eyes include inflammation of the eyelid (blepharitis), red eye caused by conjunctivitis and irritation of the eye. More rare ocular side effects include blurred vision, decreased night vision (which may be permanent), colour blindness, development of corneal opacities, inflammation of the cornea (keratitis), swelling of the optic disk (papilloedema, associated with IIH), photophobia and other visual disturbances.[5]
Mechanism of action
Isotretinoin's exact mechanism of action is unknown, but several studies have shown that isotretinoin induces apoptosis (programmatic cell death) in various cells in the body. Cell death may be instigated in the meibomian glands,[29][54] hypothalamic cells,[55] hippocampus cells[56][57] and—important for treatment of acne—in sebaceous gland cells.[58][59] Isotretinoin has a low affinity for retinoic acid receptors (RAR) and retinoid X receptors (RXR), but may be converted intracellularly to metabolites that act as agonists of RAR and RXR nuclear receptors.[4]
One study suggests the drug amplifies production of neutrophil gelatinase-associated lipocalin (NGAL) in the skin, which has been shown to reduce sebum production by inducing apoptosis in sebaceous gland cells, while exhibiting an antimicrobial effect on Propionibacterium acnes.[60][61][62] The drug decreases the size and sebum output of the sebaceous glands.[63] Isotretinoin is the only available acne drug that affects all four major pathogenic processes in acne, which distinguishes it from alternative treatments (such as antibiotics) and accounts for its efficacy in severe, nodulocystic cases.[64] The effect of Isotretinoin on sebum production can be temporary,[6] or remission of the disease can be "complete and prolonged."[63][65][66]
Isotretinoin has been speculated to down-regulate the telomerase enzyme and hTERT, inhibiting "cellular immortalization and tumorigenesis."[67] In a 2007 study, Isotretinoin was proven to inhibit the action of the metalloprotease MMP-9 (gelatinase) in sebum without any influence in the action of TIMP1 and TIMP2 (the tissue inhibitors of metalloproteases).[68] It is already known that metalloproteases play an important role in the pathogenesis of acne.[69]
Pharmacokinetics
Oral Isotretinoin is best absorbed when taken with a high-fat meal, because it has a high level of lipophilicity.[70] The efficacy of isotretinoin doubles when taken after a high-fat meal compared to when taken without food.[71] Due to Isotretinoin's molecular relationship to Vitamin A, it should not be taken with Vitamin A supplements due to the danger of toxicity through cumulative overdosing.[72] Accutane also negatively interacts with tetracycline, another class of acne drug, and with micro-dosed ('mini-pill') progesterone preparations, norethisterone/ethinylestradiol ('OrthoNovum 7/7/7'), St. John's Wort, phenytoin, and systemic corticosteroids.
Isotretinoin is primarily (99.9%) bound to plasma proteins, mostly albumin. Three metabolites of Isotretinoin are detectable in human plasma after oral administration: 4-oxo-isotretinoin, retinoid acid (tretinoin), and 4-oxo-retinoic acid (4-oxo-tretinoin). Isotretinoin also oxidizes, irreversibly, to 4-oxo-isotretinoin—which forms its geometric isomer 4-oxo-tretinoin. After an orally-administered, 80 mg dose of liquid suspension 14C-isotretinoin, 14C-activity in blood declines with a half-life of 90 hours.[70] The metabolites of isotretinoin and its conjugates are then excreted in the subject's urine and faeces in relatively equal amounts.[70] After a single, 80 mg oral dose of Isotretinoin to 74 healthy adult subjects under fed conditions, the mean ±SD elimination half-life (t1/2) of isotretinoin and 4-oxo-isotretinoin were 21.0 ± 8.2 hours and 24.0 ± 5.3 hours, respectively.[70] After both single and multiple doses, the observed accumulation ratios of isotretinoin ranged from 0.90 to 5.43 in patients with cystic acne.[70]
History
The compound 13-cis retinoic acid was first studied in the 1960s at Roche Laboratories in Switzerland by Werner Bollag as a treatment for skin cancer. Experiments completed in 1971 showed that the compound was likely to be ineffective for cancer and, surprisingly, that it could be useful to treat acne. However, they also showed that the compound was likely to cause birth defects, so in light of the events around thalidomide, Roche abandoned the product. In 1975, Gary Peck and Frank Yoder independently rediscovered the drug's use as a treatment of cystic acne while studying it as a treatment for lamellar ichthyosis, and published that work. Roche resumed work on the drug, In clinical trials, subjects were carefully screened to avoid including women who were or might become pregnant. Roche's New Drug Application for isotretinoin for the treatment of acne included data showing that the drug caused birth defects in rabbits. The FDA approved the application in 1982.
Scientists involved in the clinical trials published articles warning of birth defects at the same time the drug was launched in the US, but nonetheless isotretinoin was taken up quickly and widely, both among dermatologists and general practitioners. Cases of birth defects showed up in the first year, leading the FDA to begin publishing case reports and to Roche sending warning letters to doctors and placing warning stickers on drug bottles, and including stronger warnings on the label. Lawsuits against Roche started to be filed. In 1983 the FDA's advisory committee was convened and recommended stronger measures, which the FDA took and were that time unprecedented: warning blood banks not to accept blood from people taking the drug, and adding a warning to the label advising women to start taking contraceptives a month before starting the drug. However use of the drug continued to grow, as did the number of babies born with birth defects. In 1985 the label was updated to include a boxed warning. In early 1988 the FDA called for another advisory committee, and FDA employees prepared an internal memo estimating that around 1,000 babies had been born with birth defects due isotretinoin, that up to around 1,000 miscarriages had been caused, and that between 5,000 and 7,000 women had had abortions due to isotretinoin. The memo was leaked to the New York Times[73] a few days before the meeting, leading to a storm of media attention. In the committee meeting, dermatologists and Roche each argued to keep the drug on the market but to increase education efforts; pediatricians and the CDC argued to withdraw the drug from the market. The committee recommended to restrict physicians who could prescribe the drug and to require a second opinion before it could be prescribed. The FDA, believing it did not have authority under the law to restrict who had the right to prescribe the drug, kept the drug on the market but took further unprecedented measures: it required to Roche to make warnings yet more visible and graphic, provide doctors with informed consent forms to be used when prescribing the drug, and to conduct follow up studies to test whether the measures were reducing exposure of pregnant women to the drug. Roche implemented those measures, and offered to pay for contraception counseling and pregnancy testing for women prescribed the drug - the program was called the "Pregnancy Prevention Program".
A CDC report published in 2000[74] showed problems with the Pregnancy Prevention Program and showed that the increase in prescriptions was from off-label use, and prompted Roche to revamp its program, renaming it the "Targeted Pregnancy Prevention Program" and adding label changes like requirements for two pregnancy tests, two kinds of contraception, and for doctors to provide pharmacists with prescriptions directly; providing additional educational materials, and providing free pregnancy tests. The FDA had another advisory meeting in late 2000 that again debated how to prevent pregnant women from being exposed to the drug; dermatologists testified about the remarkable efficacy of the drug, the psychological impact of acne, and demanded autonomy to prescribe the drug; others argued that the drug be withdrawn or much stricter measures be taken. In 2001 the FDA announced a new regulatory scheme called SMART (the System to Manage Accutane Related Teratogenicity) that required Roche to provide defined training materials to doctors, and for doctors to sign and return a letter to Roche acknowledging that they had reviewed the training materials, for Roche to then send stickers to doctors, which doctors would have to place on prescriptions they give patients after they have confirmed a negative pregnancy test; prescriptions could only be written for 30 days and could not be renewed, thus requiring a new pregnancy test for each prescription.
In February 2002, Roche's patents for isotretinoin expired, and there are now many other companies selling cheaper generic versions of the drug. On June 29, 2009, Roche Pharmaceuticals, the original creator and distributor of isotretinoin, officially discontinued both the manufacture and distribution of their Accutane brand in the United States due to what the company described as business reasons related to low market share (below 5%), coupled with the high cost of defending personal-injury lawsuits brought by some patients prescribed the drug.[75] Generic isotretinoin will remain available in the United States through various manufacturers. Roche USA continues to defend Accutane and claims to have treated over 13 million patients since its introduction in 1982. F. Hoffmann-La Roche Ltd. apparently will continue to manufacture and distribute Roaccutane outside of the United States.[76]
Among others, actor James Marshall sued Roche over allegedly Accutane-related disease that resulted in removal of his colon.[77] The jury, however, decided that James Marshall had a pre-existing bowel disease.[78]
Several trials over inflammatory bowel disease claims have been held in the United States thus far, with many of them resulting in multimillion-dollar judgments against the makers of isotretinoin.[79]
Society and culture
As of 2017 isotretinoin was marketed under many brand names worldwide: A-Cnotren, Absorica, Accuran, Accutane, Accutin, Acne Free, Acnecutan, Acnegen, Acnemin, Acneone, Acneral, Acnestar, Acnetane, Acnetin A, Acnetrait, Acnetrex, Acnogen, Acnotin, Acnotren, Acretin, Actaven, Acugen, Acutret, Acutrex, Ai Si Jie, Aisoskin, Aknal, Aknefug Iso, Aknenormin, Aknesil, Aknetrent, Amnesteem, Atlacne, Atretin, Axotret, Casius, Ciscutan, Claravis, Contracné, Curacne, Curacné, Curakne, Curatane, Cuticilin, Decutan, Dercutane, Effederm, Epuris, Eudyna, Farmacne, Flexresan, Flitrion, I-Ret, Inerta, Inflader, Inotrin, Isac, Isdiben, Isoacne, Isobest, Isocural, Isoderm, Isoface, IsoGalen, Isogeril, Isolve, Isoprotil, Isoriac, Isosupra, Isosupra Lidose, Isotane, Isotina, Isotinon, Isotren, Isotret, Isotretinoin, Isotretinoina, Isotretinoína, Isotretinoine, Isotretinoïne, Isotrétinoïne, Isotretinoinum, Isotrex, Isotrin, Isotroin, Izotek, Izotziaja, Lisacne, Locatret, Mayesta, Myorisan, Neotrex, Netlook, Nimegen, Noitron, Noroseptan, Novacne, Oralne, Oraret, Oratane, Piplex, Policano, Procuta, Reducar, Retin A, Roaccutan, Roaccutane, Roacnetan, Roacta, Roacutan, Rocne, Rocta, Sotret, Stiefotrex, Tai Er Si, Teweisi, Tretin, Tretinac, Tretinex, Tretvita, Tufacne, Zenatane, Zerocutan, Zonatian ME, and Zoretanin.[1]
As of 2017 it was marketed as a topical combination drug with erythromycin under the brand names Isotrex Eritromicina, Isotrexin, and Munderm.[1]
See also
References
- 1 2 3 4 "Isotretinoin international brands". Drugs.com. Retrieved 1 June 2017.
- ↑ Millsop JW, Heller MM, Eliason MJ, Murase JE (2013). "Dermatological medication effects on male fertility". Dermatol Ther. 26 (4): 337–46. PMID 23914891. doi:10.1111/dth.12069.
- ↑ Merritt B, Burkhart CN, Morrell DS (June 2009). "Use of isotretinoin for acne vulgaris". Pediatr Ann. 38 (6): 311–20. PMID 19588674. doi:10.3928/00904481-20090512-01.
- 1 2 Layton A (May 2009). "The use of isotretinoin in acne". Dermatoendocrinol. 1 (3): 162–9. PMC 2835909
 . PMID 20436884. doi:10.4161/derm.1.3.9364.
. PMID 20436884. doi:10.4161/derm.1.3.9364. - 1 2 3 "Roaccutane 20mg Soft Capsules - Summary of Product Characteristics". UK Electronic Medicines Compendium. 1 July 2015.
- 1 2 3 US Label (PDF) (Report). FDA. 22 October 2010 [January 2010]. Retrieved 1 June 2017. See FDA Index page for NDA 018662 for updates
- ↑ Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, Thiboutot DM, Van Voorhees AS, Beutner KA, Sieck CK, Bhushan R (April 2007). "Guidelines of care for acne vulgaris management". J. Am. Acad. Dermatol. 56 (4): 651–63. PMID 17276540. doi:10.1016/j.jaad.2006.08.048.
- 1 2 Klasco RK, editor. Drugdex system, vol. 128. Greenwood Village (CO): Thomson Micromedex; 2006.
- ↑ Georgala S, Katoulis AC, Georgala C, Bozi E, Mortakis A (June 2004). "Oral isotretinoin in the treatment of recalcitrant condylomata acuminata of the cervix: a randomised placebo controlled trial". Sex Transm Infect. 80 (3): 216–8. PMC 1744851
 . PMID 15170007. doi:10.1136/sti.2003.006841.
. PMID 15170007. doi:10.1136/sti.2003.006841. - ↑ Sehgal VN, Srivastava G, Sardana K (June 2006). "Isotretinoin--unapproved indications/uses and dosage: a physician's reference". Int. J. Dermatol. 45 (6): 772–7. PMID 16796650. doi:10.1111/j.1365-4632.2006.02830.x.
- 1 2 Choi JS, Koren G, Nulman I. Pregnancy and isotretinoin therapy. CMAJ. 2013 Mar 19;185(5):411-3. doi:10.1503/cmaj.120729. PMID 23296582. PMC 3602257
- ↑ Joint Formulary Committee. British National Formulary. 47th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain. ISBN 0-85369-584-9
- ↑ "Fresh call for GPs to prescribe Roaccutane". AustralianDoctor. 19 June 2012.
- ↑ Specifically, doctors who are fellows of the Australasian College of Dermatologists (FACD); cf. Pharmaceutical Services Branch, Guide to poisons and therapeutic goods legislation for medical practitioners and dentists, Sydney: NSW Department of Health; 2006.
- ↑ James M (June 1996). "Isotretinoin for severe acne". Lancet. 347 (9017): 1749–50. PMID 8656912. doi:10.1016/S0140-6736(96)90814-4.
- ↑ "Acne, Isotretinoin, and Depression". MEDSAFE (New Zealand Ministry of Health). June 2013 [June 2005]. Retrieved 7 February 2014.
- ↑ "iPledge (About iPledge)".
- ↑ "Isotretinoin (marketed as Accutane) Capsule Information". U.S. Food and Drug Administration (FDA).
- ↑ "Isotretinoin 20 mg capsules, Summary of Product Characteristics". electronic Medicines Compendium (eMC). Datapharm Communications Ltd.
- ↑ Lawson JP, McGuire J (1987). "The spectrum of skeletal changes associated with long-term administration of 13-cis-retinoic acid". Skeletal Radiology. 16 (2): 91–7. PMID 3107131. doi:10.1007/BF00367754.
- ↑ David M, Hodak E, Lowe NJ (1988). "Adverse effects of retinoids". Medical toxicology and adverse drug experience. 3 (4): 273–88. PMID 3054426. doi:10.1007/bf03259940.
- ↑ Orfanos CE (1989). "Retinoide: der neue Stand. Erhaltungstherapie, Resorptionsstörungen bei 'non-responders', Interaktionen und Interferenzen mit Medikamenten, Behandlung von Kindern und Knochentoxizität, Acitretin und 13-cis-Acitretin" [Retinoids: the new status. Maintenance therapy, disorders of resorption in 'non-responders', interactions and interferences with drugs, treatment of children and bone toxicity, acitetin and 13-cis-acitretin]. Hautarzt (in German). 40 (3): 123–9. PMID 2523875.
- ↑ DiGiovanna JJ (2001). "Isotretinoin effects on bone". Journal of the American Academy of Dermatology. 45 (5): S176–82. PMID 11606950. doi:10.1067/mjd.2001.113721.
- 1 2 Moy A, McNamara NA, Lin MC (September 2015). "Effects of Isotretinoin on Meibomian Glands". Optometry and Vision Science. 92 (9): 925–30. PMID 26154692. doi:10.1097/OPX.0000000000000656.
- ↑ Örsal E, Seven B, Erdem MT, Varoğlu E, Farimaz H, Ayan AK. "Evaluation of the effect of isotretinoin on salivary gland function by Tc-99m pertechnetate imaging in acne vulgaris patients". Turkish Journal of Medical Sciences. 45 (3): 674–7. PMID 26281338. doi:10.3906/sag-1402-131.
- ↑ Lupi-Pégurier L, Muller-Bolla M, Fontas E, Ortonne JP. "Reduced salivary flow induced by systemic isotretinoin may lead to dental decay. A prospective clinical study". Dermatology. 214 (3): 221–6. PMID 17377383. doi:10.1159/000099586.
- ↑ Oikarinen K, Salo T, Kylmäniemi M, Palatsi R, Karhunen T, Oikarinen A (December 1995). "Systemic oral isotretinoin therapy and flow rate, pH, and matrix metalloproteinase-9 activity of stimulated saliva". Acta Odontologica Scandinavica. 53 (6): 369–71. PMID 8849870. doi:10.3109/00016359509006003.
- ↑ Reynolds NJ, Gough M, Clamp JR, Burton JL (August 1991). "Effect of oral isotretinoin therapy on saliva volume and composition". The British Journal of Dermatology. 125 (2): 189–90. PMID 1832930. doi:10.1111/j.1365-2133.1991.tb06071.x.
- 1 2 Lambert RW, Smith RE (March 1989). "Effects of 13-cis-retinoic acid on the hamster meibomian gland". J. Invest. Dermatol. 92 (3): 321–5. PMID 2918239. doi:10.1111/1523-1747.ep12277122.
- ↑ Mollan SP, Woodcock M, Siddiqi R, Huntbach J, Good P, Scott RA (August 2006). "Does use of isotretinoin rule out a career in flying?". The British Journal of Ophthalmology. 90 (8): 957–9. PMC 1857209
 . PMID 16723361. doi:10.1136/bjo.2006.092833.
. PMID 16723361. doi:10.1136/bjo.2006.092833. - ↑ Fraunfelder FT, Fraunfelder FW, Edwards R (September 2001). "Ocular side effects possibly associated with isotretinoin usage". American Journal of Ophthalmology. 132 (3): 299–305. PMID 11530040. doi:10.1016/S0002-9394(01)01024-8.
- ↑ Pittsley RA, Yoder FW (1983). "Retinoid Hyperostosis". New England Journal of Medicine. 308 (17): 1012–4. PMID 6403861. doi:10.1056/NEJM198304283081707.
- ↑ Tangrea JA, Kilcoyne RF, Taylor PR, Helsel WE, Adrianza ME, Hartman AM, Edwards BK, Peck GL (1992). "Skeletal hyperostosis in patients receiving chronic, very-low-dose isotretinoin". Archives of dermatology. 128 (7): 921–5. PMID 1626958. doi:10.1001/archderm.1992.01680170053004.
- 1 2 3 4 Brelsford, M; Beute, TC (September 2008). "Preventing and managing the side effects of isotretinoin.". Seminars in cutaneous medicine and surgery. 27 (3): 197–206. PMID 18786498. doi:10.1016/j.sder.2008.07.002.
- ↑ Scheinfeld N, Bangalore S (May 2006). "Facial edema induced by isotretinoin use: a case and a review of the side effects of isotretinoin". J Drugs Dermatol. 5 (5): 467–8. PMID 16703787.
- ↑ Roche Products Pty Ltd. Roaccutane (Australian Approved Product Information). Dee Why (NSW): Roche; 2005.
- ↑ Leyden JJ, Del Rosso JQ, Baum EW (February 2014). "The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions". J Clin Aesthet Dermatol. 7 (2 Suppl): S3–S21. PMC 3970835
 . PMID 24688620.
. PMID 24688620. - ↑ BNF, edition 57
- 1 2 Goodfield MJ, Cox NH, Bowser A, McMillan JC, Millard LG, Simpson NB, Ormerod AD (2010). "Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. 2010" (PDF). British Journal of Dermatology. 162 (6): 1172–1179. ISSN 0007-0963. PMID 21250961. doi:10.1111/j.1365-2133.2010.09836.x.
- 1 2 "Interactive Drug Analysis Profile - Isotretinoin". mhra.gov.uk. Medicines & Healthcare Products Regulatory Agency. 31 March 2017.
- 1 2 3 Kontaxakis VP, Skourides D, Ferentinos P, Havaki-Kontaxaki BJ, Papadimitriou GN (January 2009). "Isotretinoin and psychopathology: a review". Annals of General Psychiatry. 8: 2. PMC 2637283
 . PMID 19154613. doi:10.1186/1744-859X-8-2.
. PMID 19154613. doi:10.1186/1744-859X-8-2. - ↑ Wysowski, D. K.; Pitts, M.; Beitz, J. (2001-10-01). "An analysis of reports of depression and suicide in patients treated with isotretinoin". Journal of the American Academy of Dermatology. 45 (4): 515–519. ISSN 0190-9622. PMID 11568740. doi:10.1067/mjd.2001.117730.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Bremner JD, Shearer KD, McCaffery PJ (January 2012). "Retinoic acid and affective disorders: the evidence for an association". The Journal of Clinical Psychiatry (Systematic Review). 73 (1): 37–50. PMC 3276716
 . PMID 21903028. doi:10.4088/JCP.10r05993.
. PMID 21903028. doi:10.4088/JCP.10r05993. - 1 2 Rowe C, Spelman L, Oziemski M, Ryan A, Manoharan S, Wilson P, Daubney M, Scott J (May 2014). "Isotretinoin and mental health in adolescents: Australian consensus". The Australasian Journal of Dermatology (Review). 55 (2): 162–7. PMID 24283385. doi:10.1111/ajd.12117.
- ↑ Nagler, Arielle R.; Orlow, Seth J. (2015-02-01). "Dermatologists' attitudes, prescription, and counseling patterns for isotretinoin: a questionnaire-based study". Journal of drugs in dermatology: JDD. 14 (2): 184–189. ISSN 1545-9616. PMID 25689814.
- ↑ Byrne A, Costello M, Greene E, Zibin T (1998-06-01). "Isotretinoin therapy and depression – evidence for an association". Irish Journal of Psychological Medicine. 15 (2): 58–60. doi:10.1017/S0790966700003530.
- 1 2 3 4 Borovaya A, Olisova O, Ruzicka T, Sárdy M (September 2013). "Does isotretinoin therapy of acne cure or cause depression?". International Journal of Dermatology. 52 (9): 1040–52. PMID 23962262. doi:10.1111/ijd.12169.
- ↑ Gupta, M. A.; Gupta, A. K.; Schork, N. J.; Ellis, C. N.; Voorhees, J. J. (1990-12-01). "Psychiatric aspects of the treatment of mild to moderate facial acne. Some preliminary observations". International Journal of Dermatology. 29 (10): 719–721. ISSN 0011-9059. PMID 2148562.
- ↑ Magin, Parker; Pond, Dimity; Smith, Wayne (2005-02-01). "Isotretinoin, depression and suicide: a review of the evidence". Br J Gen Pract. 55 (511): 134–138. ISSN 0960-1643. PMC 1463189
 . PMID 15720936.
. PMID 15720936. - ↑ Hong Ng, Chee; Schweitzer, Isaac (2003-02-01). "The Association Between Depression and Isotretinoin Use in Acne". Australian & New Zealand Journal of Psychiatry. 37 (1): 78–84. PMID 12534661. doi:10.1046/j.1440-1614.2003.01111.x.
- 1 2 3 4 Brelsford M, Beute TC (September 2008). "Preventing and managing the side effects of isotretinoin". Seminars in Cutaneous Medicine and Surgery. 27 (3): 197–206. PMID 18786498. doi:10.1016/j.sder.2008.07.002.
- ↑ Crockett, Seth D.; Porter, Carol Q.; Martin, Christopher F.; Sandler, Robert S.; Kappelman, Michael D. (2017-05-06). "Isotretinoin Use and the Risk of Inflammatory Bowel Disease: A Case Control Study". The American journal of gastroenterology. 105 (9): 1986–1993. ISSN 0002-9270. PMC 3073620
 . PMID 20354506. doi:10.1038/ajg.2010.124.
. PMID 20354506. doi:10.1038/ajg.2010.124. - ↑ Lowenstein, Elie B.; Lowenstein, Eve J. "Isotretinoin systemic therapy and the shadow cast upon dermatology's downtrodden hero". Clinics in Dermatology. 29 (6): 652–661. doi:10.1016/j.clindermatol.2011.08.026.
- ↑ Kremer I, Gaton DD, David M, Gaton E, Shapiro A (1994). "Toxic effects of systemic retinoids on meibomian glands". Ophthalmic Res. 26 (2): 124–8. PMID 8196934. doi:10.1159/000267402.
- ↑ Griffin JN, Pinali D, Olds K, Lu N, Appleby L, Doan L, Lane MA (November 2010). "13-Cis-retinoic acid decreases hypothalamic cell number in vitro". Neurosci. Res. 68 (3): 185–90. PMID 20708044. doi:10.1016/j.neures.2010.08.003.
- ↑ Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P (April 2004). "13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice". Proc. Natl. Acad. Sci. U.S.A. 101 (14): 5111–6. Bibcode:2004PNAS..101.5111C. JSTOR 3371827. PMC 387382
 . PMID 15051884. doi:10.1073/pnas.0306336101.
. PMID 15051884. doi:10.1073/pnas.0306336101. - ↑ Sakai Y, Crandall JE, Brodsky J, McCaffery P (June 2004). "13-cis Retinoic acid (accutane) suppresses hippocampal cell survival in mice". Ann. N. Y. Acad. Sci. 1021: 436–40. Bibcode:2004NYASA1021..436S. PMID 15251924. doi:10.1196/annals.1308.059.
- ↑ Nelson AM, Cong Z, Gilliland KL, Thiboutot DM (September 2011). "TRAIL contributes to the apoptotic effect of 13-cis retinoic acid in human sebaceous gland cells". Br. J. Dermatol. 165 (3): 526–33. PMC 3166444
 . PMID 21564055. doi:10.1111/j.1365-2133.2011.10392.x.
. PMID 21564055. doi:10.1111/j.1365-2133.2011.10392.x. - ↑ Nelson AM, Gilliland KL, Cong Z, Thiboutot DM (October 2006). "13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes". J. Invest. Dermatol. 126 (10): 2178–89. PMID 16575387. doi:10.1038/sj.jid.5700289.
- ↑ Wachter K (2009). "Isotretinoin's Mechanism of Action Explored". Skin & Allergy News. 40 (11): 32. doi:10.1016/S0037-6337(09)70553-4.
- ↑ Isotretinoin’s Mechanism of Action Elucidated Archived 2010-04-04 at the Wayback Machine.. Medconnect (2009-08-28). Retrieved on 2010-11-13.
- ↑ Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM (April 2008). "Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells". J. Clin. Invest. 118 (4): 1468–78. PMC 2262030
 . PMID 18317594. doi:10.1172/JCI33869.
. PMID 18317594. doi:10.1172/JCI33869. - 1 2 Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, Butkus D, Arnaud-Battandier J (February 1979). "Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid". N. Engl. J. Med. 300 (7): 329–33. PMID 153472. doi:10.1056/NEJM197902153000701.
- ↑ Shalita A (2001). "The integral role of topical and oral retinoids in the early treatment of acne". European Academy of Dermatology and Venereology (JEADV). 15: 47. doi:10.1046/j.0926-9959.2001.00012.x.
- ↑ Farrell LN, Strauss JS, Stranieri AM (December 1980). "The treatment of severe cystic acne with 13-cis-retinoic acid. Evaluation of sebum production and the clinical response in a multiple-dose trial". J. Am. Acad. Dermatol. 3 (6): 602–11. PMID 6451637. doi:10.1016/S0190-9622(80)80074-0.
- ↑ Jones H, Blanc D, Cunliffe WJ (November 1980). "13-cis retinoic acid and acne". Lancet. 2 (8203): 1048–9. PMID 6107678. doi:10.1016/S0140-6736(80)92273-4.
- ↑ Pendino F, Flexor M, Delhommeau F, Buet D, Lanotte M, Segal-Bendirdjian E (June 2001). "Retinoids down-regulate telomerase and telomere length in a pathway distinct from leukemia cell differentiation". Proc. Natl. Acad. Sci. U.S.A. 98 (12): 6662–7. Bibcode:2001PNAS...98.6662P. JSTOR 3055868. PMC 34517
 . PMID 11371621. doi:10.1073/pnas.111464998.
. PMID 11371621. doi:10.1073/pnas.111464998. - ↑ Φαχαντίδης, Παναγιώτης Ε. (2007). Η επίδραση της ισοτρετινοϊνης και των αναστολέων της 5α-αναγωγάσης στις μεταλλοπρωτεάσες του συνδετικού ιστού σε ασθενείς με ακμή [The influence of isotretinoin and 5-a reductase inhibitors in metaloproteases of connective tissue in patients with ance] (in Greek). Aristotle University of Thessaloniki.
- ↑ Toyoda M, Nakamura M, Makino T, Kagoura M, Morohashi M (June 2002). "Sebaceous glands in acne patients express high levels of neutral endopeptidase". Exp. Dermatol. 11 (3): 241–7. PMID 12102663. doi:10.1034/j.1600-0625.2002.110307.x.
- 1 2 3 4 5 "FDA information, side effects, and uses / Accutane (isotretinoin)". U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ↑ "FDA information, side effects, and uses / Accutane (isotretinoin) : Table 2 Pharmacokinetic Parameters of Isotretinoin Mean (%CV), N=74". U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ↑ "FDA information, side effects, and uses / Accutane (isotretinoin) : Drug Interactions". U. S. Food and Drug Administration (FDA). Retrieved 20 January 2014.
- ↑ Gina Kolata for the New York Times. April 22, 1988 Anti-Acne Drug Faulted in Birth
- ↑ CDC. January 21, 2000 Accutane®-Exposed Pregnancies -- California, 1999 MMWR Weekly 49(02);28-31
- ↑ Shari Roan (7 November 2009). "New study may deal final blow to acne drug Accutane". LA Times.
- ↑ "Roche Discontinues and Plans to Delist Accutane in the U.S." (Press release). Genentech. 2009-06-29. Retrieved 2010-11-12.
- ↑ Feeley J (2011-03-11). "Roche Accutane Acne Drug Caused 'Tragedy' for Actor, Brian Dennehy Says". Bloomberg.
- ↑ Silverman E (2011-11-04). "It's Curtains On Actor's Accutane Lawsuit". Pharmalot. UBM Canon.
- ↑ Voreacos D (May 30, 2007). "Roche Found Liable in First Of 400 Suits Over Accutane". The Washington Post. Bloomberg News. Retrieved April 30, 2012.