Ataxin 1
| ATXN1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||
| |||||||||||||||
| Identifiers | |||||||||||||||
| Aliases | ATXN1, ATX1, D6S504E, SCA1, ataxin 1 | ||||||||||||||
| External IDs | OMIM: 601556 MGI: 104783 HomoloGene: 281 GeneCards: ATXN1 | ||||||||||||||
| RNA expression pattern | |||||||||||||||
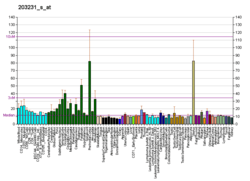 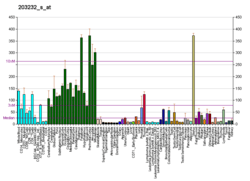 | |||||||||||||||
| More reference expression data | |||||||||||||||
| Orthologs | |||||||||||||||
| Species | Human | Mouse | |||||||||||||
| Entrez | |||||||||||||||
| Ensembl | |||||||||||||||
| UniProt | |||||||||||||||
| RefSeq (mRNA) | |||||||||||||||
| RefSeq (protein) | |||||||||||||||
| Location (UCSC) | Chr 6: 16.3 – 16.76 Mb | Chr 13: 45.55 – 45.97 Mb | |||||||||||||
| PubMed search | [1] | [2] | |||||||||||||
| Wikidata | |||||||||||||||
| |||||||||||||||
Ataxin-1 is a DNA-binding protein encoded by the ATXN1 gene.[3][4]
Mutations in this gene have been linked to neuronal death in suffers of spinocerebellar ataxia type 1, an inherited neurodegenerative disease characterized by a progressive loss of cerebellar neurons, particularly Purkinje neurons. Pathogenic ATXN1 contains an abnormally elongated stretch of the amino acid glutamine. This elongation is variable in length. The shortest repeat length shown to cause disease in humans is 39 uninterrupted CAG triplets, and the longest observed contained 83 repeats. Longer repeat tracts are correlated with earlier age of onset and faster progression.[5] How polyglutamine expansion in Ataxin-1 causes neuronal dysfunction and degeneration is still unclear. Mutant Ataxin-1 protein spontaneously misfolds and forms aggregates in the nucleus of Purkinje neurons,[6] but it has been shown in mice that aggregation is not required for pathogenesis.[7] Soluble Ataxin-1 interacts with many proteins, studies of such interactions have shown that polyglutamine expansion in Ataxin-1 causes both dominant gain-of-function and loss-of-function pathologies.[8] It has been shown that other neuronal proteins can modulate the formation of Ataxin-1 aggregates and that this in turn may affect aggregate-induced toxicity.[9]
Interactions
Ataxin 1 has been shown to interact with:
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Volz A, Fonatsch C, Ziegler A (Jun 1992). "Regional mapping of the gene for autosomal dominant spinocerebellar ataxia (SCA1) by localizing the closely linked D6S89 locus to 6p24.2----p23.05". Cytogenet Cell Genet. 60 (1): 37–9. PMID 1582256. doi:10.1159/000133291.
- ↑ "Entrez Gene: ATXN1 ataxin 1".
- ↑ Donato, Stefano Di; Mariotti, Caterina; Taroni, Franco (2012-01-01). Dürr, Sankara H. Subramony and Alexandra, ed. Handbook of Clinical Neurology. Ataxic Disorders. 103. Elsevier. pp. 399–421.
- ↑ Shastry BS. (2003). "Neurodegenerative disorders of protein aggregation.". Neurochem Int. 43 (1): 1–7. PMID 12605877. doi:10.1016/s0197-0186(02)00196-1.
- ↑ Klement, Ivan A; Skinner, Pamela J; Kaytor, Michael D; Yi, Hong; Hersch, Steven M; Clark, H.Brent; Zoghbi, Huda Y; Orr, Harry T. "Ataxin-1 Nuclear Localization and Aggregation". Cell. 95 (1): 41–53. doi:10.1016/s0092-8674(00)81781-x.
- ↑ Lim, Janghoo; Crespo-Barreto, Juan; Jafar-Nejad, Paymaan; Bowman, Aaron B.; Richman, Ronald; Hill, David E.; Orr, Harry T.; Zoghbi, Huda Y. (2008-04-10). "Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1". Nature. 452 (7188): 713–718. ISSN 0028-0836. PMC 2377396
 . PMID 18337722. doi:10.1038/nature06731.
. PMID 18337722. doi:10.1038/nature06731. - ↑ Petrakis S, Raskó T, Russ J, Friedrich RP, Stroedicke M, Riechers SP, Muehlenberg K, Möller A, Reinhardt A, Vinayagam A, Schaefer MH, Boutros M, Tricoire H, Andrade-Navarro MA, Wanker EE (Aug 2012). "Identification of human proteins that modify misfolding and proteotoxicity of pathogenic ataxin-1". PLoS Genet. 8 (8): e1002897. PMC 3420947
 . PMID 22916034. doi:10.1371/journal.pgen.1002897.
. PMID 22916034. doi:10.1371/journal.pgen.1002897. - ↑ Al-Ramahi I, Lam YC, Chen HK, de Gouyon B, Zhang M, Pérez AM, Branco J, de Haro M, Patterson C, Zoghbi HY, Botas J (2006). "CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation". J. Biol. Chem. 281 (36): 26714–24. PMID 16831871. doi:10.1074/jbc.M601603200.
- ↑ de Chiara C, Menon RP, Dal Piaz F, Calder L, Pastore A (2005). "Polyglutamine is not all: the functional role of the AXH domain in the ataxin-1 protein". J. Mol. Biol. 354 (4): 883–93. PMID 16277991. doi:10.1016/j.jmb.2005.09.083.
- ↑ Tsuda H, Jafar-Nejad H, Patel AJ, Sun Y, Chen HK, Rose MF, Venken KJ, Botas J, Orr HT, Bellen HJ, Zoghbi HY (2005). "The AXH domain of Ataxin-1 mediates neurodegeneration through its interaction with Gfi-1/Senseless proteins". Cell. 122 (4): 633–44. PMID 16122429. doi:10.1016/j.cell.2005.06.012.
- ↑ Mizutani A, Wang L, Rajan H, Vig PJ, Alaynick WA, Thaler JP, Tsai CC (2005). "Boat, an AXH domain protein, suppresses the cytotoxicity of mutant ataxin-1". EMBO J. 24 (18): 3339–51. PMC 1224676
 . PMID 16121196. doi:10.1038/sj.emboj.7600785.
. PMID 16121196. doi:10.1038/sj.emboj.7600785. - ↑ Park Y, Hong S, Kim SJ, Kang S (2005). "Proteasome function is inhibited by polyglutamine-expanded ataxin-1, the SCA1 gene product". Mol. Cells. 19 (1): 23–30. PMID 15750336.
- ↑ Irwin S, Vandelft M, Pinchev D, Howell JL, Graczyk J, Orr HT, Truant R (2005). "RNA association and nucleocytoplasmic shuttling by ataxin-1". J. Cell. Sci. 118 (Pt 1): 233–42. PMID 15615787. doi:10.1242/jcs.01611.
- ↑ Suter, B.; Fontaine, J.-F.; Yildirimman, R.; Rasko, T.; Schaefer, M. H.; Rasche, A.; Porras, P.; Vazquez-Alvarez, B. M.; Russ, J.; Rau, K.; Foulle, R.; Zenkner, M.; Saar, K.; Herwig, R.; Andrade-Navarro, M. A.; Wanker, E. E. (28 December 2012). "Development and application of a DNA microarray-based yeast two-hybrid system". Nucleic Acids Research. pp. 1496–1507. doi:10.1093/nar/gks1329.
- ↑ Hong S, Ka S, Kim S, Park Y, Kang S (May 2003). "p80 coilin, a coiled body-specific protein, interacts with ataxin-1, the SCA1 gene product". Biochim. Biophys. Acta. 1638 (1): 35–42. PMID 12757932. doi:10.1016/s0925-4439(03)00038-3.
- 1 2 Hong S, Lee S, Cho SG, Kang S (Jun 2008). "UbcH6 interacts with and ubiquitinates the SCA1 gene product ataxin-1". Biochem. Biophys. Res. Commun. 371 (2): 256–60. PMID 18439907. doi:10.1016/j.bbrc.2008.04.066.
- ↑ Koshy B, Matilla T, Burright EN, Merry DE, Fischbeck KH, Orr HT, Zoghbi HY (Sep 1996). "Spinocerebellar ataxia type-1 and spinobulbar muscular atrophy gene products interact with glyceraldehyde-3-phosphate dehydrogenase". Hum. Mol. Genet. 5 (9): 1311–8. PMID 8872471. doi:10.1093/hmg/5.9.1311.
- ↑ Hong S, Kim SJ, Ka S, Choi I, Kang S (Jun 2002). "USP7, a ubiquitin-specific protease, interacts with ataxin-1, the SCA1 gene product". Mol. Cell. Neurosci. 20 (2): 298–306. PMID 12093161. doi:10.1006/mcne.2002.1103.
External links
- GeneReviews/NIH/NCBI/UW entry on Spinocerebellar Ataxia Type 1
- ataxin-1 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human ATXN1 genome location and ATXN1 gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.