ADAR
Double-stranded RNA-specific adenosine deaminase is an enzyme that in humans is encoded by the ADAR gene (which stands for adenosine deaminase acting on RNA).[3][4]
Adenosine deaminases acting on RNA (ADAR) are enzymes responsible for binding to double stranded RNA (dsRNA) and converting adenosine (A) to inosine (I) by deamination.[5] ADAR protein is a RNA-binding protein, which functions in RNA-editing through post-transcriptional modification of mRNA transcripts by changing the nucleotide content of the RNA.[6] The conversion from A to I in the RNA disrupt the normal A:U pairing which makes the RNA unstable. Inosine is structurally similar to that of guanine (G) which leads to I to cytosine (C) binding. In RNA I functions the same as G in both translation and replication. Codon changes can arise from editing which may lead to changes in the coding sequences for proteins and their functions.[7] Most editing site are found in noncoding regions of RNA such as untranslated regions (UTRs), Alu elements and long interspersed nuclear element (LINEs). Mutations in this gene have been associated with dyschromatosis symmetrica hereditaria, as well as Aicardi–Goutières syndrome.[8] Alternate transcriptional splice variants, encoding different isoforms, have been characterized.[6]
Discovery
Adenosine Deaminase Acting on RNA (ADAR) and its gene were first discovered accidentally in 1987 as a result of research by Brenda Bass and Harold Weintraub.[9] These researchers were using antisense RNA inhibition to determine which genes play a key role in the development of Xenopus leavis embryos. Previous research on Xenopus oocytes had been successful. However, when Bass and Weintraub applied identical protocols to Xenopus embryos, they were unable to determine the embryo’s developmental genes. In an attempt to understand why the method was unsuccessful, they began comparing duplex RNA in both oocytes and embryos. This lead them to discover that a developmentally regulated activity denatures RNA:RNA hybrids in embryos.
In 1988, Richard Wagner et al. further studied the activity occurring on Xenopus embryos.[10] They determined that a protein was responsible for the unwinding of RNA due to the absence of activity after proteinase treatment. It was also shown that this protein is specific for double stranded RNA, or dsRNA, and does not require ATP. Additionally, it became evident that the protein’s activity on dsRNA modifies it beyond a point of rehybridization, but does not fully denature it. Finally, the researchers determined that this unwinding is due to the deamination of adenosine residues to inosine. This modification results in mismatched base-pairing between inosine and uridine, leading to the destabilization and unwinding of dsRNA.
Function and origin
Adenosine Deaminase Acting on RNA is one of the most common forms of RNA editing, and has both selective and non-selective activity.[11] ADAR is able to both modify and regulate the output of gene product, as inosine is interpreted by the cell to be guanosine. ADAR has also been determined to change the functionality of small RNA molecules. Its is believed that ADAR evolved from ADAT (Adenosine Deaminase Acting on tRNA), a critical protein present in all eukaryotes, early in the metazoan period through the addition of a dsRNA binding domain. This likely occurred in the lineage which leads to the crown Metazoa when a duplicate ADAT gene was coupled to a gene encoding at least one double stranded RNA binding. The ADAR family of genes has been largely conserved over the history of its existence. This, along with its presence in the majority of modern phyla, indicates that RNA editing is an essential regulatory gene for metazoan organisms. ADAR has not been discovered in a variety of non-metazoan eukaryotes, such as plants, fungi and choanoflagellates.
Types
In mammals, there are three types of ADARs, 1, 2 and 3.[12] ADAR1 and ADAR2 are found in many tissues in the body while ADAR3 is only found in the brain.[7] ADAR1 and ADAR2 are known to be catalytically active while ADAR3 is thought to be inactive.[7] ADAR1 has two known isoforms known as ADAR1p150 and ADAR1p110. ADAR1p110 is only found in the nucleus and ADAR1p150 goes from the nucleus to the cytoplasm.[12]
Catalytic activity
Biochemical reaction
ADARs catalyze the reaction from A to I by hydrolytic deamination.[5] It does this by the use of an activated water molecule for a nucelophilic attack. It is done by the addition of water to carbon 6 and removal of ammonia with a hydrated intermediate.
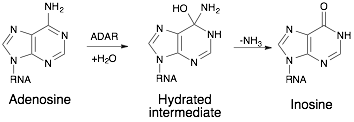
Active site
In humans, the enzyme's active site has 2-3 amino-terminal dsRNA binding domains (dsRBDs) and one carboxy terminal catalytic deaminase domain.[12] In the dsRBD domain there is a conserved α-β-β-β-α configuration present.[7] ADAR1 contains two areas for binding Z-DNA known as Zα and Zβ. ADAR2 and ADAR3 have an arginine rich single stranded RNA (ssRNA) binding domain. A crystal structure of ADAR2 has been solved.[12] In the enzyme active site, there is a glutamic acid residue(E396) that hydrogen bonds to a water. There is a histidine (H394) and two cysteine restudies (C451 and C516) that coordinates a zinc ion. The zinc activates the water molecule for the nucelophilic hydrolytic deamination. Within the catalytic core there is an inositol hexakisphosphate (IP6), which stabilizes arginine and lysine residues.
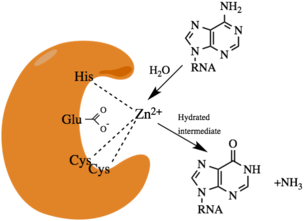
Dimerization
It has been found in mammals that the conversion from A to I requires homodimerization of ADAR1 and ADAR2, but not ADAR3.[7] In vivo studies have not yet been conclusive if RNA binding is required for dimerization. A study with ADAR1 and 2 mutants which were not able to bind to dsRNA were still able to dimerize, showing they may bind based on protein-protein interactions [7][13]
Model organisms
Model organisms have been used in the study of ADAR function. A conditional knockout mouse line, called Adartm1a(EUCOMM)Wtsi[14][15] was generated as part of the International Knockout Mouse Consortium program — a high-throughput mutagenesis project to generate and distribute animal models of disease to interested scientists[16][17][18] Male and female animals underwent a standardized phenotypic screen to determine the effects of deletion.[19][20] Twenty five tests were carried out on mutant mice and two significant abnormalities were observed.[6] Few homozygous mutant embryos were identified during gestation, and none survived until weaning. The remaining tests were carried out on heterozygous mutant adult mice and no abnormalities were observed in these animals.[19]
Role in disease
Aicardi–Goutières Syndrome
ADAR1 is one of multiple genes which can contribute to Aicardi–Goutières syndrome when mutated.[8] This is a genetic inflammatory disease primarily affecting the skin and the brain. The inflammation is caused by incorrect activation of interferon inducible genes such as those activated to fight off viral infections. Mutation and loss of function of ADAR1 prevents destabilization of double stranded RNA (dsRNA) and the body mistakes this for viral RNA resulting in an autoimmune response.[21]
HIV
Research has shown that ADAR1 can be both beneficial and a hindrance in a cells ability to fight off HIV infection. Expression levels of the ADAR1 protein have shown to be elevated during HIV infection and it has been suggested that it is responsible for A to G mutations in the HIV genome, inhibiting replication.[22] The authors of this study also suggest that mutation of the HIV genome by ADAR1 might in some cases lead to beneficial viral mutations which could contribute to drug resistance.
Hepatocellular carcinoma
Studies of samples from patients with hepatocellular carcinoma (HCC) have shown that ADAR1 is frequently upregulated and ADAR2 is frequently downregulated in the disease. It has been suggested that this is responsible for the disrupted A to I editing pattern seen in HCC and that ADAR1 acts as an oncogene in this context whilst ADAR2 has tumor suppressor activities.[23] The imbalance of ADAR expression could change the frequency of A to I transitions in the protein coding region of genes, resulting in mutated proteins which drive the disease. The dysregulation of ADAR1 and ADAR2 could be used as a possible poor prognostic marker.
Melanoma
In contrast to hepatocellular carcinoma, several research studies have indicated that loss of ADAR1 contributes to melanoma growth and metastasis. It is known that ADAR can act on microRNA and affect it’s biogenesis, stability and/or it’s binding target.[24] It has been suggested that ADAR1 is downregulated by cAMP- response element binding protein (CREB), limiting its ability to act on miRNA.[25] One such example is miR-455-5p which is edited by ADAR1. When ADAR is downregulated by CREB the unedited miR-455-5p downregulates a tumor suppressor protein called CPEB1, contributing to melanoma progression in an in vivo model.[25]
Dyschromatosis Symmetrica Hereditaria (DSH1)
A Gly1007Arg mutation in ADAR1, as well as other truncated versions, have been implicated as a cause in some cases of DSH1.[26] This is a disease characterized by hyperpigmentation in the hands and feet and can occur in Japanese and Chinese families.
Viral activity
Antiviral
ADAR1 is an interferon ( IFN )-inducible protein (one released by a cell in response to a pathogen or virus), so it would make sense that it would assist with a cell’s immune pathway. This seems to be true for the HCV replicon, Lymphocytic choriomeningitis LCMV, and polyomavirus [27]
Proviral
ADAR1 is known to be proviral in other circumstances. ADAR1’s A to I editing has been found in many viruses including measles virus,[28][29] influenza virus,[30] lymphocytic choriomeningitis virus,[31] polyomavirus,[32] hepatitis delta virus,[33] and hepatitis C virus.[34] Although ADAR1 has been seen in other viruses, it has only been studied extensively in a few; one of those is measles virus (MV). Research done on MV has shown that ADAR1 enhances viral replication. This is done through two different mechanisms: RNA editing and inhibition of dsRNA-activated protein kinase (PKR).[27] Specifically, viruses are thought to use ADAR1 as a positive replication factor by selectively suppressing dsRNA-dependent and antiviral pathways.[35]
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K (November 1994). "Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing". Proceedings of the National Academy of Sciences of the United States of America. 91 (24): 11457–61. PMC 45250
 . PMID 7972084. doi:10.1073/pnas.91.24.11457.
. PMID 7972084. doi:10.1073/pnas.91.24.11457. - ↑ "Entrez Gene: ADAR Adenosine Deaminase Acting on RNA".
- 1 2 Samuel CE (2012). Adenosine deaminases acting on RNA (ADARs) and A-to-I editing. Heidelberg: Springer. ISBN 978-3-642-22800-1.
- 1 2 "ADAR". NBCI. U.S. National Library of Medicine.
- 1 2 3 4 5 6 Nishikura K (7 June 2010). "Functions and regulation of RNA editing by ADAR deaminases". Annual Review of Biochemistry. 79 (1): 321–49. PMC 2953425
 . PMID 20192758. doi:10.1146/annurev-biochem-060208-105251.
. PMID 20192758. doi:10.1146/annurev-biochem-060208-105251. - 1 2 Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, et al. (November 2012). "Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature". Nature Genetics. 44 (11): 1243–8. PMC 4154508
 . PMID 23001123. doi:10.1038/ng.2414.
. PMID 23001123. doi:10.1038/ng.2414. - ↑ Samuel CE (March 2011). "Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral". Virology. 411 (2): 180–93. PMC 3057271
 . PMID 21211811. doi:10.1016/j.virol.2010.12.004.
. PMID 21211811. doi:10.1016/j.virol.2010.12.004. - ↑ Wagner RW, Smith JE, Cooperman BS, Nishikura K (1989). "A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs". Proceedings of the National Academy of Sciences of the United States of America. 86 (8): 2647–51. PMC 286974
 . PMID 2704740. doi:10.1073/pnas.86.8.2647.
. PMID 2704740. doi:10.1073/pnas.86.8.2647. - ↑ Grice LF, Degnan BM (2015-01-29). "The origin of the ADAR gene family and animal RNA editing". BMC Evolutionary Biology. 15 (1): 4. PMC 4323055
 . PMID 25630791. doi:10.1186/s12862-015-0279-3.
. PMID 25630791. doi:10.1186/s12862-015-0279-3. - 1 2 3 4 Savva YA, Rieder LE, Reenan RA (2012). "The ADAR protein family". Genome Biology. 13 (12): 252. PMC 3580408
 . PMID 23273215. doi:10.1186/gb-2012-13-12-252.
. PMID 23273215. doi:10.1186/gb-2012-13-12-252. - ↑ Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K (May 2003). "Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA". The Journal of Biological Chemistry. 278 (19): 17093–102. PMID 12618436. doi:10.1074/jbc.M213127200.
- ↑ "International Knockout Mouse Consortium".
- ↑ "Mouse Genome Informatics".
- ↑ Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A (June 2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–42. PMC 3572410
 . PMID 21677750. doi:10.1038/nature10163.
. PMID 21677750. doi:10.1038/nature10163. - ↑ Dolgin E (June 2011). "Mouse library set to be knockout". Nature. 474 (7351): 262–3. PMID 21677718. doi:10.1038/474262a.
- ↑ Collins FS, Rossant J, Wurst W (January 2007). "A mouse for all reasons". Cell. 128 (1): 9–13. PMID 17218247. doi:10.1016/j.cell.2006.12.018.
- 1 2 GERDIN, AK (September 2010). "The Sanger Mouse Genetics Programme: high throughput characterisation of knockout mice". Acta Ophthalmologica. 88: 0–0. doi:10.1111/j.1755-3768.2010.4142.x.
- ↑ van der Weyden L, White JK, Adams DJ, Logan DW (2011). "The mouse genetics toolkit: revealing function and mechanism". Genome Biology. 12 (6): 224. PMC 3218837
 . PMID 21722353. doi:10.1186/gb-2011-12-6-224.
. PMID 21722353. doi:10.1186/gb-2011-12-6-224. - ↑ Liddicoat BJ, Piskol R, Chalk AM, Ramaswami G, Higuchi M, Hartner JC, Li JB, Seeburg PH, Walkley CR (September 2015). "RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself". Science. 349 (6252): 1115–20. PMID 26275108. doi:10.1126/science.aac7049.
- ↑ Weiden MD, Hoshino S, Levy DN, Li Y, Kumar R, Burke SA, Dawson R, Hioe CE, Borkowsky W, Rom WN, Hoshino Y (2014). "Adenosine deaminase acting on RNA-1 (ADAR1) inhibits HIV-1 replication in human alveolar macrophages". PloS One. 9 (10): e108476. PMC 4182706
 . PMID 25272020. doi:10.1371/journal.pone.0108476.
. PMID 25272020. doi:10.1371/journal.pone.0108476. - ↑ Chan TH, Lin CH, Qi L, Fei J, Li Y, Yong KJ, Liu M, Song Y, Chow RK, Ng VH, Yuan YF, Tenen DG, Guan XY, Chen L (May 2014). "A disrupted RNA editing balance mediated by ADARs (Adenosine DeAminases that act on RNA) in human hepatocellular carcinoma". Gut. 63 (5): 832–43. PMC 3995272
 . PMID 23766440. doi:10.1136/gutjnl-2012-304037.
. PMID 23766440. doi:10.1136/gutjnl-2012-304037. - ↑ Heale BS, Keegan LP, McGurk L, Michlewski G, Brindle J, Stanton CM, Caceres JF, O'Connell MA (October 2009). "Editing independent effects of ADARs on the miRNA/siRNA pathways". The EMBO Journal. 28 (20): 3145–56. PMC 2735678
 . PMID 19713932. doi:10.1038/emboj.2009.244.
. PMID 19713932. doi:10.1038/emboj.2009.244. - 1 2 Shoshan E, Mobley AK, Braeuer RR, Kamiya T, Huang L, Vasquez ME, Salameh A, Lee HJ, Kim SJ, Ivan C, Velazquez-Torres G, Nip KM, Zhu K, Brooks D, Jones SJ, Birol I, Mosqueda M, Wen YY, Eterovic AK, Sood AK, Hwu P, Gershenwald JE, Robertson AG, Calin GA, Markel G, Fidler IJ, Bar-Eli M (March 2015). "Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis". Nature Cell Biology. 17 (3): 311–21. PMC 4344852
 . PMID 25686251. doi:10.1038/ncb3110.
. PMID 25686251. doi:10.1038/ncb3110. - ↑ Tojo K, Sekijima Y, Suzuki T, Suzuki N, Tomita Y, Yoshida K, Hashimoto T, Ikeda S (September 2006). "Dystonia, mental deterioration, and dyschromatosis symmetrica hereditaria in a family with ADAR1 mutation". Movement Disorders. 21 (9): 1510–3. PMID 16817193. doi:10.1002/mds.21011.
- 1 2 Gélinas JF, Clerzius G, Shaw E, Gatignol A (September 2011). "Enhancement of replication of RNA viruses by ADAR1 via RNA editing and inhibition of RNA-activated protein kinase". Journal of Virology. 85 (17): 8460–6. PMC 3165853
 . PMID 21490091. doi:10.1128/JVI.00240-11.
. PMID 21490091. doi:10.1128/JVI.00240-11. - ↑ Baczko K, Lampe J, Liebert UG, Brinckmann U, ter Meulen V, Pardowitz I, Budka H, Cosby SL, Isserte S, Rima BK (November 1993). "Clonal expansion of hypermutated measles virus in a SSPE brain". Virology. 197 (1): 188–95. PMID 8212553. doi:10.1006/viro.1993.1579.
- ↑ Cattaneo (21 October 1988). "Biased hypermutation and other genetic changes in defective measles viruses in human brain infections". Cell. 55 (2): 255–65. doi:10.1016/0092-8674(88)90048-7.
- ↑ Tenoever BR, Ng SL, Chua MA, McWhirter SM, García-Sastre A, Maniatis T (March 2007). "Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity". Science. 315 (5816): 1274–8. PMID 17332413. doi:10.1126/science.1136567.
- ↑ Zahn RC, Schelp I, Utermöhlen O, von Laer D (January 2007). "A-to-G hypermutation in the genome of lymphocytic choriomeningitis virus". Journal of Virology. 81 (2): 457–64. PMC 1797460
 . PMID 17020943. doi:10.1128/jvi.00067-06.
. PMID 17020943. doi:10.1128/jvi.00067-06. - ↑ Kumar (15 April 1997). "Nuclear antisense RNA induces extensive adenosine modifications and nuclear retention of target transcripts". Proc Natl Acad Sci USA. 94 (8): 3542–7. doi:10.1073/pnas.94.8.3542.
- ↑ Luo GX, Chao M, Hsieh SY, Sureau C, Nishikura K, Taylor J (1990). "A specific base transition occurs on replicating hepatitis delta virus RNA". Journal of Virology. 64 (3): 1021–7. PMC 249212
 . PMID 2304136.
. PMID 2304136. - ↑ Taylor DR, Puig M, Darnell ME, Mihalik K, Feinstone SM (2005). "New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1". Journal of Virology. 79 (10): 6291–8. PMC 1091666
 . PMID 15858013. doi:10.1128/JVI.79.10.6291-6298.2005.
. PMID 15858013. doi:10.1128/JVI.79.10.6291-6298.2005. - ↑ Toth AM, Li Z, Cattaneo R, Samuel CE (October 2009). "RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR". The Journal of Biological Chemistry. 284 (43): 29350–6. PMC 2785566
 . PMID 19710021. doi:10.1074/jbc.M109.045146.
. PMID 19710021. doi:10.1074/jbc.M109.045146.
Further reading
- Valenzuela A, Blanco J, Callebaut C, Jacotot E, Lluis C, Hovanessian AG, Franco R (1997). "HIV-1 envelope gp120 and viral particles block adenosine deaminase binding to human CD26". Advances in Experimental Medicine and Biology. 421: 185–92. PMID 9330696. doi:10.1007/978-1-4757-9613-1_24.
- Wathelet MG, Szpirer J, Nols CB, Clauss IM, De Wit L, Islam MQ, Levan G, Horisberger MA, Content J, Szpirer C (September 1988). "Cloning and chromosomal location of human genes inducible by type I interferon". Somatic Cell and Molecular Genetics. 14 (5): 415–26. PMID 3175763. doi:10.1007/BF01534709.
- Wang Y, Zeng Y, Murray JM, Nishikura K (November 1995). "Genomic organization and chromosomal location of the human dsRNA adenosine deaminase gene: the enzyme for glutamate-activated ion channel RNA editing". Journal of Molecular Biology. 254 (2): 184–95. PMID 7490742. doi:10.1006/jmbi.1995.0610.
- Patterson JB, Samuel CE (October 1995). "Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase". Molecular and Cellular Biology. 15 (10): 5376–88. PMC 230787
 . PMID 7565688. doi:10.1128/mcb.15.10.5376.
. PMID 7565688. doi:10.1128/mcb.15.10.5376. - Patterson JB, Thomis DC, Hans SL, Samuel CE (July 1995). "Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons". Virology. 210 (2): 508–11. PMID 7618288. doi:10.1006/viro.1995.1370.
- O'Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W (March 1995). "Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase". Molecular and Cellular Biology. 15 (3): 1389–97. PMC 230363
 . PMID 7862132. doi:10.1128/mcb.15.3.1389.
. PMID 7862132. doi:10.1128/mcb.15.3.1389. - Weier HU, George CX, Greulich KM, Samuel CE (November 1995). "The interferon-inducible, double-stranded RNA-specific adenosine deaminase gene (DSRAD) maps to human chromosome 1q21.1-21.2". Genomics. 30 (2): 372–5. PMID 8586444. doi:10.1006/geno.1995.0034.
- Liu Y, George CX, Patterson JB, Samuel CE (February 1997). "Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase". The Journal of Biological Chemistry. 272 (7): 4419–28. PMID 9020165. doi:10.1074/jbc.272.7.4419.
- Valenzuela A, Blanco J, Callebaut C, Jacotot E, Lluis C, Hovanessian AG, Franco R (April 1997). "Adenosine deaminase binding to human CD26 is inhibited by HIV-1 envelope glycoprotein gp120 and viral particles". Journal of Immunology. 158 (8): 3721–9. PMID 9103436.
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A (August 1997). "A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase". Proceedings of the National Academy of Sciences of the United States of America. 94 (16): 8421–6. PMC 22942
 . PMID 9237992. doi:10.1073/pnas.94.16.8421.
. PMID 9237992. doi:10.1073/pnas.94.16.8421. - Liu Y, Herbert A, Rich A, Samuel CE (July 1998). "Double-stranded RNA-specific adenosine deaminase: nucleic acid binding properties". Methods. 15 (3): 199–205. PMID 9735305. doi:10.1006/meth.1998.0624.
- George CX, Samuel CE (April 1999). "Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible". Proceedings of the National Academy of Sciences of the United States of America. 96 (8): 4621–6. PMC 16382
 . PMID 10200312. doi:10.1073/pnas.96.8.4621.
. PMID 10200312. doi:10.1073/pnas.96.8.4621. - Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A (June 1999). "Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA". Science. 284 (5421): 1841–5. PMID 10364558. doi:10.1126/science.284.5421.1841.
- Schade M, Turner CJ, Kühne R, Schmieder P, Lowenhaupt K, Herbert A, Rich A, Oschkinat H (October 1999). "The solution structure of the Zalpha domain of the human RNA editing enzyme ADAR1 reveals a prepositioned binding surface for Z-DNA". Proceedings of the National Academy of Sciences of the United States of America. 96 (22): 12465–70. PMC 22950
 . PMID 10535945. doi:10.1073/pnas.96.22.12465.
. PMID 10535945. doi:10.1073/pnas.96.22.12465. - Blanco J, Valenzuela A, Herrera C, Lluís C, Hovanessian AG, Franco R (July 2000). "The HIV-1 gp120 inhibits the binding of adenosine deaminase to CD26 by a mechanism modulated by CD4 and CXCR4 expression". FEBS Letters. 477 (1–2): 123–8. PMID 10899322. doi:10.1016/S0014-5793(00)01751-8.
- Herrera C, Morimoto C, Blanco J, Mallol J, Arenzana F, Lluis C, Franco R (June 2001). "Comodulation of CXCR4 and CD26 in human lymphocytes". The Journal of Biological Chemistry. 276 (22): 19532–9. PMID 11278278. doi:10.1074/jbc.M004586200.
- Wong SK, Sato S, Lazinski DW (June 2001). "Substrate recognition by ADAR1 and ADAR2". RNA. 7 (6): 846–58. PMC 1370134
 . PMID 11421361. doi:10.1017/S135583820101007X.
. PMID 11421361. doi:10.1017/S135583820101007X. - Eckmann CR, Neunteufl A, Pfaffstetter L, Jantsch MF (July 2001). "The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein". Molecular Biology of the Cell. 12 (7): 1911–24. PMC 55639
 . PMID 11451992. doi:10.1091/mbc.12.7.1911.
. PMID 11451992. doi:10.1091/mbc.12.7.1911.
External links
- OMIM entries on Dyschromatosis Symmetrica Hereditaria 1
- ADAR human gene location in the UCSC Genome Browser.
- ADAR human gene details in the UCSC Genome Browser.