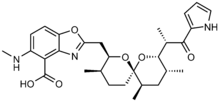
A23187
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| ECHA InfoCard | 100.052.786 |
| Chemical and physical data | |
| Formula | C29H37N3O6 |
| Molar mass | 523.62 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
A23187 is a mobile ion-carrier that forms stable complexes with divalent cations (ions with a charge of +2). A23187 is also known as Calcimycin, Calcium Ionophore, Antibiotic A23187 and Calcium Ionophore A23187. It is produced at fermentation of Streptomyces chartreusensis.
Actions and uses
A23187 has antibiotic properties against gram positive bacteria and fungi. It also acts as a divalent cation ionophore, allowing these ions to cross cell membranes, which are usually impermeable to them.[1] A23187 is most selective for Mn2+, somewhat less selective for Ca2+ and Mg2+, much less selective for Sr2+, and even less selective for Ba2+.[2] The ionophore is used in laboratories to increase intracellular Ca2+ levels in intact cells. It also uncouples oxidative phosphorylation, the process cells use to synthesize Adenosine triphosphate which they use for energy. In addition, A23187 inhibits mitochondrial ATPase activity. A23187 also induces apoptosis in some cells (e.g. mouse lymphoma cell line, or S49, and Jurkat cells) and prevents it in others (e.g. cells dependent on interleukin 3 that have had the factor withdrawn).[3]
Inex Pharmaceuticals Corporation (Canada) reported an innovative application of A23187. Inex used A23187 as a molecular tool in order to make artificial liposomes loaded with anti-cancer drugs such as Topotecan.[4]
In IVF field, Ca Ionophore can be used in case of low fertilization rate after ICSI procedure, particularly with Globozoospermia (Round Head sperm syndrome), Ca Ionophore will replace absence of sperm acrosom, and plays role in oocyte activation after ICSI. Recommended use is 0.5 microgram/ml twice for 10 min interrupted with fresh media with 30 min incubation, followed with regular injected eggs culture for IVF.[5]
Biosynthesis
The core biosynthetic enzymes are thought to include 3 proteins for the biosynthesis of the α-ketopyrrole moiety, 5 for modular type I polyketide synthases for the spiroketal ring, 4 for the biosynthesis of 3-hydroxyanthranilic acid, an N-methyltransferase tailoring enzyme, and a type II thioesterase.[6]
Commercial availability
Commercially, A23187 is available as free acid, Ca2+ salt, and 4-brominated analog.[7]
References
- ↑ "A23187". Medical Dictionary Online. 2006. Archived from the original on 10 December 2006. Retrieved 2006-11-01.
- ↑ Abbott, Bernard; D S Fukuda; D E Dorman; J L Occolowitz; M Debono; L Farhner (1979). "Microbial transformation of A23187, a divalent cation ionophore antibiotic". Antimicrobial Agents and Chemotherapy. 16 (6): 808–812. PMC 352958
 . PMID 119484. doi:10.1128/aac.16.6.808.
. PMID 119484. doi:10.1128/aac.16.6.808. - ↑ "A23187". Archived from the original on 3 November 2006. Retrieved 2006-11-01.
- ↑ Liposomal Encapsulation of Topotecan Enhances Anticancer Efficacy in Murine and Human Xenograft Models Cancer Research
- ↑ Effect of calcium ionophore on unfertilized oocytes after ICSI cycles; Iran J Reprod Med Vol. 10. No. 2. pp: 83-86, March 2012.
- ↑ Wu Q, Liang J, Lin S, Zhou X, Bai L, Deng Z, Wang Z (March 2011). "Characterization of the biosynthesis gene cluster for the pyrrole polyether antibiotic calcimycin (A23187) in Streptomyces chartreusis NRRL 3882". Antimicrob Agents Chemother. 55 (3): 974–82. PMC 3067094
 . PMID 21173184. doi:10.1128/AAC.01130-10.
. PMID 21173184. doi:10.1128/AAC.01130-10. - ↑ A23187 Archived June 26, 2006, at the Wayback Machine. product page from Fermentek
External links
- A23187 from AG Scientific, another vendor
- A21387 from BIOMOL, a vendor's product page
- Calcimycin from Bioaustralis, a vendor's product page