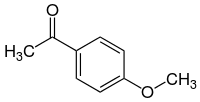
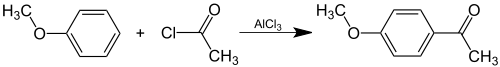Acetanisole
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4-Methoxyphenyl)ethan-1-one | |
| Other names
4-Acetylanisole; para-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.560 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.18 g·mol−1 |
| Appearance | White to pale yellow crystals[1] |
| Density | 1.094 g/cm3 |
| Melting point | 38.5 °C (101.3 °F; 311.6 K)[2] |
| Boiling point | 258 °C (496 °F; 531 K)[2] |
| 2470 mg/L[2] | |
| Hazards | |
| Flash point | 138 °C (280 °F)[3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acetanisole is an aromatic chemical compound with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition Acetanisole can sometimes smell like butter or caramel. [3] It is used as a cigarette additive,[4] a fragrance,[1] and a flavoring in food.[5]
Acetanisole is found naturally in castoreum, the glandular secretion of the beaver.[1]
Acetanisole can be prepared synthetically by Friedel-Crafts acylation of anisole with acetyl chloride:
Appearance
At room temperature 4-Methoxyacetophenone is solid, and has a white crystal like structure. Once melted, the white crystals turn into a clear liquid.
References
- 1 2 3 Para-Acetanisole, The Good Scents Company
- 1 2 3 Acetanisole in the ChemIDplus database
- 1 2 Acetanisole at Sigma-Aldrich
- ↑ Tobacco Documents | Profiles | Additives | Acetanisole Archived April 11, 2008, at the Wayback Machine.
- ↑ 21 C.F.R. 172.515
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.