3-dehydroquinate synthase
| 3-dehydroquinate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
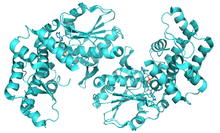 Ribbon representation of the Helicobacter pylori 3-dehydroquinate synthase.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 4.2.3.4 | ||||||||
| CAS number | 37211-77-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| 3-dehydroquinate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | DHQ_synthase | ||||||||
| Pfam | PF01761 | ||||||||
| InterPro | IPR002658 | ||||||||
| SCOP | 1dqs | ||||||||
| SUPERFAMILY | 1dqs | ||||||||
| |||||||||
In enzymology, a 3-dehydroquinate synthase (EC 4.2.3.4) is an enzyme that catalyzes the chemical reaction
Hence, this enzyme has one substrate, 3-deoxy-arabino-heptulosonate 7-phosphate, and two products, 3-dehydroquinate and phosphate. The protein uses NAD+ to catalyze the reaction.[2][3] This reaction is part of the shikimate pathway which is involved in the biosynthesis of aromatic amino acids.
3-Dehydroquinate synthase belongs to the family of lyases, to be specific those carbon-oxygen lyases acting on phosphates. This enzyme participates in phenylalanine, tyrosine, and tryptophan biosynthesis. It employs one cofactor, cobalt.

Background
The shikimate pathway is composed of seven steps, each catalyzed by an enzyme. The shikimate pathway is responsible for producing the precursors for aromatic amino acids, which are essential to our diets because we cannot synthesize them in our bodies. Only plants, bacteria, and microbial eukaryotes are capable of producing aromatic amino acids. The pathway ultimately converts phosphoenolpyruvate and 4-erythrose phosphate into chorismate, the precursor to aromatic amino acids. 3-Dehydroquinate synthase is the enzyme that catalyzes reaction in the second step of this pathway. This second step of the reaction eliminates a phosphate from 3-deoxy-D-arabino-heptulosonate 7-phosphate, which results in 3-dehydroquinate. 3-Dehydroquinate synthase is a monomeric enzyme, and has a molecular weight of 39,000.[4] 3-dehydroquinate synthase is activated by inorganic phosphate, and requires NAD+ for activity, although the reaction in total is neutral when catalyzed by an enzyme.[4]
Function
3-Dehydroquinate synthase utilizes a complex multi-step mechanism that includes alcohol oxidation, phosphate b-elimination, carbonyl reduction, ring opening, and intramolecular aldol condensation.[5] Dehydroquinate synthase requires NAD+ and a cobalt cofactor to catalyze the conversion of 3-deoxy-D-arabino-heptulosonate 7-phosphate into 3-dehydroquinate. In most bacteria, this enzyme has only one function. However, in some organisms, it forms a complex with other enzymes. This complex is known as the AROM complex. The AROM complex is a pentafunctional polypeptide, which contains enzymes that catalyze steps two, three, four, and five of the shikimate pathway.[5] In addition, dehydroquinate synthase is of particular interest because of its complicated activity relative to its small size.[5]
Applications
3-Dehydroquinate synthase catalyzes the second step in the shikimate pathway, which is essential for the production of aromatic amino acids in bacteria, plants, and fungi, but not mammals. This makes it an ideal target for new antimicrobial agents, anti-parasitic agents, and herbicides.[1] Other enzymes in the shikimate pathway have already been targeted and put to use as herbicides. Roundup, a common herbicide made by Monsanto, works by inhibiting another enzyme in the shikimate pathway. The shikimate pathway is an ideal choice for herbicides because this pathway does not exist in animals or people so people are not directly affected. Roundup uses an enzyme inhibitor, glyphosate, to block one of the steps of the shikimate pathway. Glyphosate inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSP synthase), which ultimately blocks the production of aromatic amino acids, and, without aromatic amino acids, plants cannot survive. However, Monsanto developed a bacterial form of EPSP synthase that was not inhibited by Roundup. Monsanto introduced this gene into plants using agrobacterium and the result was a plant that was resistant to Roundup. This meant that all plants without the bacterial gene would die, leading a much higher degree of weed control.


Nomenclature
The systematic name of this enzyme class is 3-deoxy-arabino-heptulonate-7-phosphate phosphate-lyase (cyclizing 3-dehydroquinate-forming). Other names in common use include 5-dehydroquinate synthase, 5-dehydroquinic acid synthetase, dehydroquinate synthase, 3-dehydroquinate synthetase, 3-deoxy-arabino-heptulosonate-7-phosphate phosphate-lyase, (cyclizing), and 3-deoxy-arabino-heptulonate-7-phosphate phosphate-lyase (cyclizing).
References
- 1 2 PDB: 3CLH; Liu JS, Cheng WC, Wang HJ, Chen YC, Wang WC (August 2008). "Structure-based inhibitor discovery of Helicobacter pylori dehydroquinate synthase". Biochemical and Biophysical Research Communications. 373 (1): 1–7. PMID 18503755. doi:10.1016/j.bbrc.2008.05.070.; rendered with MacPyMOL
- ↑ Hawkins AR, Lamb HK (August 1995). "The molecular biology of multidomain proteins. Selected examples". European Journal of Biochemistry / FEBS. 232 (1): 7–18. PMID 7556173. doi:10.1111/j.1432-1033.1995.tb20775.x.
- ↑ Barten R, Meyer TF (April 1998). "Cloning and characterisation of the Neisseria gonorrhoeae aroB gene". Molecular & General Genetics. 258 (1-2): 34–44. PMID 9613570. doi:10.1007/s004380050704.
- 1 2 Herrmann KM, Weaver LM (June 1999). "The Shikimate Pathway". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 473–503. PMID 15012217. doi:10.1146/annurev.arplant.50.1.473.
- 1 2 3 Negron L, Patchett ML, Parker EJ (2011). "Expression, Purification, and Characterisation of Dehydroquinate Synthase from Pyrococcus furiosus". Enzyme Research. 2011: 134893. PMC 3092513
 . PMID 21603259. doi:10.4061/2011/134893.
. PMID 21603259. doi:10.4061/2011/134893.
Further reading
- Rotenberg SL, Sprinson DB (December 1970). "Mechanism and stereochemistry of 5-dehydroquinate synthetase". Proceedings of the National Academy of Sciences of the United States of America. 67 (4): 1669–72. PMC 283410
 . PMID 5275368. doi:10.1073/pnas.67.4.1669.
. PMID 5275368. doi:10.1073/pnas.67.4.1669. - Srinivasan PR, Rothschild J, Sprinson DB (October 1963). "The enzymic conversion of 3-deoxy-d-arabino-heptulosonic acid 7-phosphate to 5-dehydroquinate". The Journal of Biological Chemistry. 238: 3176–82. PMID 14085358.
- Bender SL, Mehdi S, Knowles JR (September 1989). "Dehydroquinate synthase: the role of divalent metal cations and of nicotinamide adenine dinucleotide in catalysis". Biochemistry. 28 (19): 7555–60. PMID 2514789. doi:10.1021/bi00445a009.
- Carpenter EP, Hawkins AR, Frost JW, Brown KA (July 1998). "Structure of dehydroquinate synthase reveals an active site capable of multistep catalysis". Nature. 394 (6690): 299–302. PMID 9685163. doi:10.1038/28431.