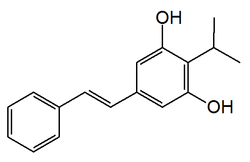
3,5-Dihydroxy-4-isopropyl-''trans''-stilbene
 | |
| Names | |
|---|---|
| IUPAC name
5-(2-Phenylethenyl)-2-propan-2-ylbenzene-1,3-diol | |
| Other names
(E)-3,5-Dihydroxy-4-isopropyl-trans-stilbene 3,5-Dihydroxy-4-isopropylstilbene | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| |
| Properties | |
| C17H18O2 | |
| Molar mass | 254.33 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,5-Dihydroxy-4-isopropyl-trans-stilbene is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes. It is a product of an alternative ketosynthase-directed stibenoids biosynthesis pathway. It is derived from the condensation of two β-ketoacyl thioesters .[1] It is produced by the Photorhabdus luminescens bacterial symbiont species of the entomopathogenic nematode, Heterorhabditis megidis. Experiments with infected larvae of Galleria mellonella, the wax moth, support the hypothesis that the compound has antibiotic properties that help minimize competition from other microorganisms and prevents the putrefaction of the nematode-infected insect cadaver.[2]

Entomopathogenic nematodes emerging from a wax moth cadaver
See also
- Pinosylvin, a molecule produced in pines that does not bear the isopropyl alkylation.
References
- ↑ Joyce SA; Brachmann AO; Glazer I; Lango L; Schwär G; Clarke DJ; Bode HB (2008). "Bacterial biosynthesis of a multipotent stilbene". Angew Chem Int Ed Engl. 47 (10): 1942–5. PMID 18236486. doi:10.1002/anie.200705148.
- ↑ Hu, K; Webster, JM (2000). "Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus--Heterorhabditis infected Galleria mellonella larvae". FEMS microbiology letters. 189 (2): 219–23. PMID 10930742. doi:10.1111/j.1574-6968.2000.tb09234.x.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.