3,3',5,5'-Tetramethylbenzidine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,3',5,5'-Tetramethyl[1,1'-biphenyl]-4,4'-diamine | |
| Other names
3,3',5,5'-Tetramethylbiphenyl-4,4'-diamine | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.053.949 |
| |
| |
| Properties | |
| C16H20N2 | |
| Molar mass | 240.3482 g/mol |
| Melting point | 168 to 171 °C (334 to 340 °F; 441 to 444 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3,3’,5,5’-Tetramethylbenzidine or TMB is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA).[1] TMB is a white crystal powder that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights.
Enzymatic assay
TMB can act as a hydrogen donor for the reduction of hydrogen peroxide to water by peroxidase enzymes such as horseradish peroxidase.
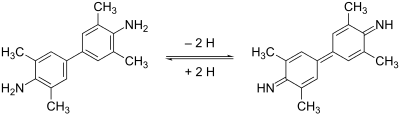
The resulting diimine causes the solution to take on a blue colour, and this colour change can be read on a spectrophotometer at a wavelength of 650 nm.
The reaction can be halted by addition of acid or another stop reagent. Using sulfuric acid turns TMB yellow. The colour may be read at 450 nm.[2]
Material Safety
TMB should be kept out of direct sunlight as it is photosensitive. It is not known if TMB is carcinogenic and the evidence is contradictory: TMB is not mutagenic by the Ames test,[3][4][5][6] and did not induce formation of tumors in a single-arm study of 24 rats.[3] On that evidence, it has been used as a replacement for carcinogenic compounds such as benzidine[7] and o-phenylenediamine.
References
- ↑ Sigma Aldrich Catalog Entry for 3,3′,5,5′-Tetramethylbenzidine
- ↑ Martin TL; Mufson EJ; Mesulam MM (1984). "The light side of horseradish peroxidase histochemistry". J Histochem Cytochem. 32 (7): 793. doi:10.1177/32.7.6736628.
- 1 2 Holland VR; Saunders BC; Rose FL; Walpole AL (1974). "A safer substitute for benzidine in the detection of blood". Tetrahedron. 30: 3299. doi:10.1016/S0040-4020(01)97504-0.
- ↑ Ashby J; Paton D; Lefevre PA; Styles JA; Rose FL (1982). "Evaluation of two suggested methods of deactivating organic carcinogens by molecular modification". Carcinogenesis. 3 (11): 1277–1282. PMID 6758975. doi:10.1093/carcin/3.11.1277.
- ↑ Chung K-T; Chen S-C; Wong TY; Li YS; Wei CI; Chou MW (2000). "Mutagenicity studies of benzidine and its analogs: Structure-activity relationships". Toxicol Sci. 56 (2): 351–356. PMID 10910993. doi:10.1093/toxsci/56.2.351.
- ↑ Chung K-T; Chen S-C; Claxton LD (2006). "Review of the Salmonella typhimurium mutagenicity of benzidine, benzidine analogues, and benzidine-based dyes". Mutation Research/Reviews in Mutation Research. 612 (1): 58–76. PMID 16198141. doi:10.1016/j.mrrev.2005.08.001.
- ↑ Yang J; Wang H; Zhang H (2008). "One-pot synthesis of silver nanoplates and charge-transfer complex nanofibers". J Phys Chem C. 112 (34): 13065–13069. doi:10.1021/jp802604d.