2.2.2-Cryptand
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane[1] | |||
| Other names
Cryptating agent 222 | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| Abbreviations | Crypt-222 | ||
| 620282 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.041.770 | ||
| EC Number | 245-962-4 | ||
| MeSH | Cryptating+agent+222 | ||
| PubChem CID |
|||
| RTECS number | MP4750000 | ||
| |||
| |||
| Properties | |||
| C 18N 2H 36O 6 | |||
| Molar mass | 376.4882 g mol−1 | ||
| Melting point | 68 to 71 °C (154 to 160 °F; 341 to 344 K) | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS signal word | WARNING | ||
| H315, H319, H335 | |||
| P261, P305+351+338 | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||

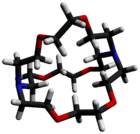
Structure of a [2.2.2]cryptand encapsulating a potassium cation (purple). At crystalline state, obtained with an X-ray diffraction.[2]
2.2.2-Cryptand is one of the most important members of the cryptand family of chelating agents. In Nomenclature of Inorganic Chemistry (2005), IUPAC recommends the abbreviation "crypt-222".
References
- ↑ "cryptating agent 222 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. Descriptors Computed from Structure.
- ↑ Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. (2001). "Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3]+". J. Am. Chem. Soc. 121 (13): 3135–3136. doi:10.1021/ja003932b.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.