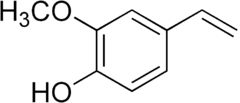
2-Methoxy-4-vinylphenol
 | |
| Names | |
|---|---|
| IUPAC name
4-Ethenyl-2-methoxyphenol | |
| Other names
4-Hydroxy-3-methoxystyrene 4-Vinylguaiacol p-Vinylguaiacol p-Vinicatechol-o-methyl ether | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.029.183 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.18 g·mol−1 |
| Boiling point | 224 °C (435 °F; 497 K) |
| Hazards | |
| Flash point | 113 °C (235 °F; 386 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2-Methoxy-4-vinylphenol is an aromatic substance used as a flavoring agent.[1] It is one of the compounds responsible for the natural aroma of buckwheat.[2]
Some insects such as Rhynchophorus ferrugineus (Red palm weevil) use this substance for chemical signaling (pheromones).[3]
The aroma of pure substance was described as: apple, spicy, peanut, wine-like or clove and curry.
Ferulic acid is converted by certain strains of yeast, notably strains used in brewing of wheat beers, such as Torulaspora delbrueckii to 2-methoxy-4-vinylphenol which gives beers such as Weissbier and Wit their distinctive "clove" flavor. Saccharomyces cerevisiae (dry brewer's yeast) and Pseudomonas fluorescens are also able to convert trans-ferulic acid into 2-methoxy-4-vinylphenol.[4]
References
- ↑ 2-METHOXY-4-VINYLPHENOL, Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives
- ↑ Janes D, Kantar D, Kreft S, Prosen H (2008). "Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC-MS". Food Chemistry. 112: 120–124. doi:10.1016/j.foodchem.2008.05.048.
- ↑ Semiochemical - 2-methoxy-4-vinylphenol, Pherobase.com
- ↑ Huang, Z.; Dostal, L.; Rosazza, J. P. (1993). "Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens". Applied and Environmental Microbiology. 59 (7): 2244–2250. PMC 182264
 . PMID 8395165.
. PMID 8395165.