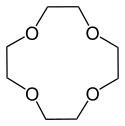
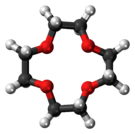
12-Crown-4
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,4,7,10-tetraoxacyclododecane | |||
| Other names
12-crown-4, Lithium Ionophore V | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.488 | ||
| |||
| |||
| Properties | |||
| C8H16O4 | |||
| Molar mass | 176.21 | ||
| Density | 1.089 g/mL at 25 °C | ||
| Melting point | 16 °C | ||
| Boiling point | 61-70 °C/0.5 mm Hg | ||
| Miscible | |||
| Hazards | |||
| Flash point | 113 °C (235 °F; 386 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
12-Crown-4, also called 1,4,7,10-tetraoxacyclododecane and lithium ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation. Its point group is S4. The dipole moment of 12-crown-4 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 12-crown-4 was determined as 2.33 ± 0.03 D in cyclohexane and 2.46 ± 0.01 D in benzene.[1]
lithium-cation-from-xtal-3D-balls-B.png)
References
- ↑ Caswell, Lyman R.; Savannunt, Diana S. (January 1988). "Temperature and solvent effects on the experimental dipole moments of three crown ethers". Journal of Heterocyclic Chemistry. 25 (1): 73–79. doi:10.1002/jhet.5570250111.
- Sigma-Aldrich Handbook of Fine Chemicals, 2007, page 768.
See also
- Crown ether
- Cyclen, a similar molecule with N atoms (aza groups) instead of O atoms (ethers)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.