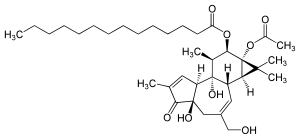
12-''O''-Tetradecanoylphorbol-13-acetate
 | |
| Names | |
|---|---|
| IUPAC name
(1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9a-(acetyloxy)-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1a,1b,4,4a,5,7a,7b,8,9,9a-decahydro-H-cyclopropa[3,4]benzo[1,2-e]azulen-9-yl myristate | |
| Other names
TPA, PMA, Phorbol myristate acetate, Tetradecanoylphorbol acetate. | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.109.485 |
| KEGG | |
| PubChem CID |
|
| |
| |
| Properties | |
| C36H56O8 | |
| Molar mass | 616.83 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
12-O-Tetradecanoylphorbol-13-acetate (TPA), also commonly known as tetradecanoylphorbol acetate, tetradecanoyl phorbol acetate, and phorbol 12-myristate 13-acetate (PMA), is a diester of phorbol and a potent tumor promoter often employed in biomedical research to activate the signal transduction enzyme protein kinase C (PKC).[1][2][3] The effects of TPA on PKC result from its similarity to one of the natural activators of classic PKC isoforms, diacylglycerol. TPA is a small molecule drug.
In ROS biology, superoxide was identified as the major reactive oxygen species induced by TPA/PMA but not by ionomycin in mouse macrophages.[4] Thus, TPA/PMA has been routinely used as an inducer for endogenous superoxide production.[5]
TPA is also being studied as a drug in the treatment of hematologic cancer
TPA has a specific use in cancer diagnostics as a B-cell specific mitogen in cytogenetic testing. To view the chromosomes, a cytogenetic test requires dividing cells. TPA is used to stimulate division of B-cells during cytogenetic diagnosis of B-cell cancers such as chronic lymphocytic leukemia.[6]
TPA is also commonly used together with ionomycin to stimulate T-cell activation, proliferation, and cytokine production, and is used in protocols for intracellular staining of these cytokines.[7]
TPA induces KSHV reactivation in PEL cell cultures via stimulation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. The pathway involves the activation of the early-immediate viral protein RTA that contributes to the activation of the lytic cycle.[8]
TPA was first found in the croton plant, a shrub found in Southeast Asia, exposure to which provokes a poison ivy-like rash. It underwent a phase 1 clinical trial.
References
- ↑ Castagna (1982). "Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters" (PDF). Journal of Biological Chemistry. 257 (13): 7847–7851. PMID 7085651.
- ↑ Blumberg (1988). "Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture". Cancer Research. 48: 1–8. PMID 3275491.
- ↑ Niedel (1983). "Phorbol Diester Receptor Copurifies with Protein Kinase C". Proceedings of the National Academy of Sciences. 80: 36–40. Bibcode:1983PNAS...80...36N. doi:10.1073/pnas.80.1.36.
- ↑ Swindle (2002). "A Comparison of Reactive Oxygen Species Generation by Rat Peritoneal Macrophages and Mast Cells Using the Highly Sensitive Real-Time Chemiluminescent Probe Pholasin: Inhibition of Antigen-Induced Mast Cell Degranulation by Macrophage-Derived Hydrogen Peroxide" (PDF). The Journal of Immunology. 169 (10): 5866–5873. doi:10.4049/jimmunol.169.10.5866.
- ↑ Huang (2014). "Megakaryocytic Differentiation of K562 Cells Induced by PMA Reduced the Activity of Respiratory Chain Complex IV". Plos One. 9 (5): e96246. doi:10.1371/journal.pone.0096246.
- ↑ The AGT cytogenetics laboratory manual. 3rd ed. Barch, Margaret J., Knutsen, Turid., Spurbeck, Jack L., eds. 1997. Lippincott-Raven.
- ↑ "Flow Cytometry Intracellular Staining Guide". eBioscience, Inc. Retrieved 2011-09-25.
- ↑ Cohen, Adina; Brodie, Chaya; Sarid, Ronit (April 2006). "An essential role of ERK signalling in TPA-induced reactivation of Kaposi's sarcoma-associated herpesvirus.". The Journal of general virology. 87 (Pt 4): 795–802. PMID 16528027. doi:10.1099/vir.0.81619-0.
External links
- Tetradecanoylphorbol Acetate at the US National Library of Medicine Medical Subject Headings (MeSH)
- "NCI Dictionary Entry". Retrieved 2005-07-02.
- "Phase 1 Clinical Trials". Retrieved 2005-07-02.