Benzisoxazole
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Benzisoxazole | |||
| Other names
Benzo[d]isoxazole; Indoxazine | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID |
|||
| |||
| |||
| Properties | |||
| C7H5NO | |||
| Molar mass | 119.12 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.18 g/cm3 | ||
| Boiling point | 35 to 38 °C (95 to 100 °F; 308 to 311 K) (at 2.67 hPa) 101-102 °C (at 2 kPa) | ||
| Hazards | |||
| S-phrases (outdated) | S24/25, S28A, S37, S45 | ||
| Flash point | 58 °C (136 °F; 331 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure.[1][2] The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
Its aromaticity makes it relatively stable;[3] however, it is only weakly basic.
Synthesis
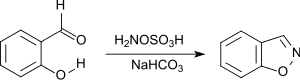
Benzisoxazole may be prepared from inexpensive salicylaldehyde, via a base catalyzed room temperature reaction with hydroxylamine-O-sulfonic acid.[4]
See also
- Structural isomers
References
- ↑ Katritzky, A. R.; Pozharskii, A. F. (2000). Handbook of Heterocyclic Chemistry (2nd ed.). Academic Press. ISBN 0080429882.
- ↑ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. (2001). Organic Chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.
- ↑ Domene, Carmen; Jenneskens, Leonardus W.; Fowler, Patrick W. (2005). "Aromaticity of anthranil and its isomers, 1,2-benzisoxazole and benzoxazole". Tetrahedron Letters. 46 (23): 4077–4080. ISSN 0040-4039. doi:10.1016/j.tetlet.2005.04.014.
- ↑ Kemp, D.S.; Woodward, R.B. (1965). "The N-ethylbenzisoxazolium cation—I". Tetrahedron. 21 (11): 3019–3035. ISSN 0040-4020. doi:10.1016/S0040-4020(01)96921-2.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.