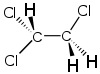
1,1,2-Trichloroethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,2-Trichloroethane | |||
| Other names
1,1,2-TCA vinyl trichloride beta-trichloroethane | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.061 | ||
| KEGG | |||
| PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H3Cl3 | |||
| Molar mass | 133.40 g/mol | ||
| Appearance | colorless liquid[1] | ||
| Odor | sweet, chloroform-like[1] | ||
| Density | 1.435 g/cm3 | ||
| Melting point | −37 °C (−35 °F; 236 K) | ||
| Boiling point | 110 to 115 °C (230 to 239 °F; 383 to 388 K) | ||
| 0.4% (20°C)[1] | |||
| Vapor pressure | 19 mmHg (20°C)[1] | ||
| Hazards | |||
| NFPA 704 | |||
| Explosive limits | 6%-15.5%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
| LCLo (lowest published) |
13,100 mg/m3 (cat, 4.5 hr) 2000 ppm (rat, 4 hr)[2] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 10 ppm (45 mg/m3) [skin][1] | ||
| REL (Recommended) |
Ca TWA 10 ppm (45 mg/m3) [skin][1] | ||
| IDLH (Immediate danger) |
Ca [100 ppm][1] | ||
| Related compounds | |||
| Related compounds |
1,1,1-Trichloroethane; Trichloroethylene | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula C2H3Cl3. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane.
It is used as a solvent and as an intermediate in the synthesis of 1,1-dichloroethane.
1,1,2-TCA is a central nervous system depressant and inhalation of vapors may cause dizziness, drowsiness, headache, nausea, shortness of breath, unconsciousness, or cancer.
Toxicology
Trichloroethane may be harmful by inhalation, ingestion and skin contact. It is a respiratory and eye irritant. Although no definitive studies currently exist, trichloroethane should be treated as a potential carcinogen since laboratory evidence suggests that low molecular weight chlorinated hydrocarbons may be carcinogenic.[3]
The Occupational Safety and Health Administration and National Institute for Occupational Safety and Health have set occupational exposure limits to 1,1,2-Trichloroethane at 10 ppm over an eight-hour time-weighted average.[4] It is considered to be a potential occupational carcinogen.
References
- 1 2 3 4 5 6 7 8 "NIOSH Pocket Guide to Chemical Hazards #0628". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "1,1,2-Trichloroethane". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Safety (MSDS) data for 1,1,2-trichloroethane" (PDF).
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards