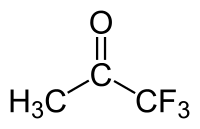
Trifluoroacetone
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1-Trifluoropropan-2-one | |
| Other names
Trifluoracetone, TFA | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.370 |
| EC Number | 207-005-9 |
| PubChem CID |
|
| |
| |
| Properties | |
| C3H3F3O | |
| Molar mass | 112.05 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.252 g/mL |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 21–24 °C (70–75 °F; 294–297 K) |
| Hazards | |
| GHS pictograms |   |
| GHS signal word | DANGER |
| H224, H315, H319, H335 | |
| P210, P261, P303, P351, P338 | |
| Flash point | −30 °C (−22 °F; 243 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trifluoroacetone (1,1,1-trifluoroacetone) is an organofluorine derivative of acetone with the chemical formula C
3H
3F
3O.[1] In contrast to fluoroacetone, the compound has three fluorine atoms. Under normal conditions, the compound is a colorless liquid with chloroform-like odour.[2]
Properties
At room temperature, trifluoroacetone is a colorless liquid. In contrast to the less volatile fluoroacetone, the compound boils starting at 22 °C. Trifluoroacetone is highly flammable because of its low boiling point. The vapors of trifluoroacetone may form an explosive mixture with air if heated above the flash point, so there has to be sufficient ventilation of the area.
Applications
Trifluoroacetone can be used as oxidizing agent in Oppenauer oxidation, in which case hydroxyl groups of secondary alcohols can be oxidized in the presence of hydroxy groups of primary alcohols.[3]
Trifluoracetone is also used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine is used to prepare enantiopure α-trifluoromethyl alanines and diamines via a Strecker reaction followed by either nitrile hydrolysis or reduction.[4]
See also
References
- ↑ "1,1,1-Trifluoracetone 95%". dk.vwr.com. Retrieved 6 June 2017.
- ↑ "1,1,1-Trifluoroacetone". Sigma Aldrich. sigmaaldrich.com. Retrieved 6 June 2017.
- ↑ Mello, Rossella; Martínez-Ferrer, Jaime; Asensio, Gregorio; González-Núñez, María Elena (2007). "Oppenauer Oxidation of Secondary Alcohols with 1,1,1-Trifluoroacetone as Hydride Acceptor". J. Org. Chem. 24 (72): 9376–9378. doi:10.1021/jo7016422. Retrieved 6 June 2017.
- ↑ "Concise synthesis of enantiopure alpha-trifluoromethyl alanines, diamines, and amino alcohols via the Strecker-type reaction.". sigmaaldrich.com. Retrieved 6 June 2017.