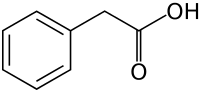
Phenylacetic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Phenylacetic acid | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.862 |
| UNII | |
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.15 g/mol |
| Density | 1.0809 g/cm3 |
| Melting point | 76 to 77 °C (169 to 171 °F; 349 to 350 K) |
| Boiling point | 265.5 °C (509.9 °F; 538.6 K) |
| 15 g/L | |
| Acidity (pKa) | 4.31[1] |
| -82.72·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phenylacetic acid (PAA) (conjugate base phenylacetate), also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a disagreeable odor. Endogeneously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone (used in the manufacture of substituted amphetamines), it is subject to controls in countries including the United States and China.[2]
Names
Synonyms include α-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid, and β-phenylacetic acid.
Occurrence
Phenylacetic acid has been found to be an active auxin (a type of plant hormone),[3] found predominantly in fruits. However, its effect is much weaker than the effect of the basic auxin molecule indole-3-acetic acid. In addition the molecule is naturally produced by the metapleural gland of most ant species and used as an antimicrobial. It is also the oxidation product of phenethylamine in humans following metabolism by monoamine oxidase and subsequent metabolism of the intermediate product, phenylacetaldehyde, by the aldehyde dehydrogenase enzyme; these enzymes are also found in many other organisms.
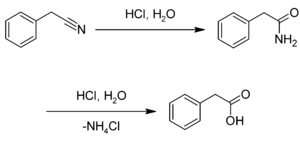
Preparation
This compound may be prepared by the hydrolysis of benzyl cyanide:[4][5]
Applications
Phenylacetic acid is used in some perfumes, as it possesses a honey-like odor in low concentrations. It is also used in penicillin G production and diclofenac production. It is also employed to treat type II hyperammonemia to help reduce the amounts of ammonia in a patient's bloodstream by forming phenylacetyl-CoA, which then reacts with nitrogen-rich glutamine to form phenylacetylglutamine. This compound is then secreted by the patient's body. It's also used in the illicit production of phenylacetone, which is used in the manufacture of methamphetamine.
Drug uses
- Camylofin is made from BnCO2H in a 3-step reaction consisting of a Hell–Volhard–Zelinsky halogenation, esterfication of the acid bromide with isoamylalcohol, and then reaction of the remaining alkylbromide with 2-(dethylamino)ethylamine.
- The reaction of phenylacetic acid with o-Phenylenediamine is used to make Bendazol.
- Another use is in the synthesis of Triafungin.
- Halogenation of phenylacetic acid to phenylacetyl chloride and amidation with urea give the phenacemide product.
See also
References
- ↑ Dippy, J. F. J.; Hughes, S. R. C.; Rozanski, A. (1959). "The dissociation constants of some symmetrically disubstituted succinic acids". Journal of the Chemical Society. 1959: 2492–2498. doi:10.1039/JR9590002492.
- ↑ "List of Regulated Drug Precursor Chemicals in China". Retrieved 27 April 2015.
- ↑ Wightman, F.; Lighty, D. L. (1982). "Identification of phenylacetic acid as a natural auxin in the shoots of higher plants". Physiologia Plantarum. 55 (1): 17–24. doi:10.1111/j.1399-3054.1982.tb00278.x.
- ↑ Adams R.; Thal, A. F. (1922). "Phenylacetic acid". Org. Synth. 2: 59.; Coll. Vol., 1, p. 436
- ↑ Wenner, W. (1952). "Phenylacetamide". Org. Synth. 32: 92.; Coll. Vol., 4, p. 760