Zingerone
 | |
| Names | |
|---|---|
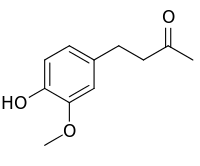
| IUPAC name
4-(4-hydroxy-3-methoxyphenyl)-2-butanone | |
| Identifiers | |
| 122-48-5 | |
| ChEBI | CHEBI:68657 |
| ChEMBL | ChEMBL25894 |
| ChemSpider | 28952 |
| Jmol interactive 3D | Image |
| PubChem | 31211 |
| UNII | 4MMW850892 |
| |
| |
| Properties | |
| C11H14O3 | |
| Molar mass | 194.22 g/mol |
| Melting point | 40 to 41 °C (104 to 106 °F; 313 to 314 K) |
| Boiling point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) at 14 mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Zingerone, also called vanillylacetone, is a key component of the pungency of ginger.[1] Zingerone is a crystalline solid that is sparingly soluble in water, but soluble in ether.[2]
Zingerone is similar in chemical structure to other flavor chemicals such as vanillin and eugenol. It is used as a flavor additive in spice oils and in perfumery to introduce spicy aromas.
Fresh ginger does not contain zingerone; cooking the ginger transforms gingerol, which is present, into zingerone through a retro-aldol reaction (reversal of aldol addition).
Ginger compounds have been shown to be active against enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea. This type of diarrhea is the leading cause of infant death in developing countries. Zingerone is likely the active constituent responsible for the antidiarrheal efficacy of ginger.[3]
References
- ↑ Monge, P; Scheline, R; Solheim, E (1976). "The metabolism of zingerone, a pungent principle of ginger". Xenobiotica 6 (7): 411–23. doi:10.3109/00498257609151654. PMID 997589.
- ↑ Steffen Arctander, Perfume and Flavor Materials of Natural Origin, pg. 280
- ↑ Chen, Jaw-Chyun; Li-Jiau Huang; Shih-Lu Wu; Sheng-Chu Kuo; Tin-Yun Ho; Chien-Yun Hsiang (2007). "Ginger and Its Bioactive Component Inhibit Enterotoxigenic Escherichia coli Heat-Labile Enterotoxin-Induced Diarrhea in Mice". Journal of Agricultural and Food Chemistry 55 (21): 8390–7. doi:10.1021/jf071460f. PMID 17880155.