Sphalerite
| Sphalerite | |
|---|---|
|
Sphalerite on dolomite from the Tri-State District, Jasper County, Missouri, USA | |
| General | |
| Category | Sulfide mineral |
| Formula (repeating unit) | (Zn,Fe)S |
| Strunz classification | 02.CB.05a |
| Dana classification | 02.08.02.01 |
| Identification | |
| Color | Brown, yellow, red, green, black. |
| Crystal habit | Euhedral crystals – occurs as well-formed crystals showing good external form. Granular – generally occurs as anhedral to subhedral crystals in matrix. |
| Crystal system | Isometric hextetrahedral (4 3m) |
| Twinning | Simple contact twins or complex lamellar forms, twin axis [111] |
| Cleavage | perfect |
| Fracture | Uneven to conchoidal |
| Mohs scale hardness | 3.5-4 |
| Luster | Adamantine, resinous, greasy |
| Streak | brownish white, pale yellow |
| Diaphaneity | Transparent to translucent, opaque when iron-rich |
| Specific gravity | 3.9–4.2 |
| Optical properties | Isotropic |
| Refractive index | nα = 2.369 |
| Other characteristics | non-radioactive, non-magnetic, fluorescent and triboluminescent. |
| References | [1][2][3] |
Sphalerite ((Zn,Fe)S) is a mineral that is the chief ore of zinc. It consists largely of zinc sulfide in crystalline form but almost always contains variable iron. When iron content is high it is an opaque black variety, marmatite. It is usually found in association with galena, pyrite, and other sulfides along with calcite, dolomite, and fluorite. Miners have also been known to refer to sphalerite as zinc blende, black-jack, and ruby jack.
Chemistry
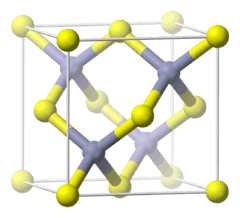 The crystal structure of sphalerite |
The mineral crystallizes in the cubic crystal system. In the crystal structure, zinc and sulfur atoms are tetrahedrally coordinated. The structure is closely related to the structure of diamond. The hexagonal analog is known as the wurtzite structure. The lattice constant for zinc sulfide in the zinc blende crystal structure is 0.541 nm,[4] calculated from geometry and ionic radii of 0.074 nm (zinc) and 0.184 nm (sulfide). It forms ABCABC layers.
Varieties

Its color is usually yellow, brown, or gray to gray-black, and it may be shiny or dull. Its luster is adamantine, resinous to submetallic for high iron varieties. It has a yellow or light brown streak, a Mohs hardness of 3.5–4, and a specific gravity of 3.9–4.1. Some specimens have a red iridescence within the gray-black crystals; these are called "ruby sphalerite." The pale yellow and red varieties have very little iron and are translucent. The darker, more opaque varieties contain more iron. Some specimens are also fluorescent in ultraviolet light. The refractive index of sphalerite (as measured via sodium light, 589.3 nm) is 2.37. Sphalerite crystallizes in the isometric crystal system and possesses perfect dodecahedral cleavage. Gemmy, pale specimens from Franklin, New Jersey (see Franklin Furnace), are highly fluorescent orange and/or blue under longwave ultraviolet light and are known as cleiophane, an almost pure ZnS variety.
Occurrence
Sphalerite is the major ore of zinc and is found in thousands of locations worldwide.[2]
Sources of high quality crystals include:[3]
- Freiberg, Saxony, and Neudorf, Harz Mountains of Germany
- The Lengenbach Quarry, Binntal, Valais, Switzerland, has produced colorless crystals.
- Horni Slavkov (Schlaggenwald) and Pribram, Czech Republic
- From Rodna, Romania
- Transparent green to opaque black Madan, Smolyan Province, Rhodope Mountains, Bulgaria;
- Transparent crystals in the Aliva mine, Picos de Europa Mountains, Cantabria [Santander] Province, Spain
- In England, from Alston Moor, Cumbria
- At Dalnegorsk, Primorskiy Kray, Russia
- In Canada
- Watson Lake, Yukon Territory
- Gord Cowie at Hudson Bay Mining and Smelting processes Sphalerite in Flin Flon, Manitoba
- In the USA
- the Tri-State district including deposits near Baxter Springs, Cherokee County, Kansas; Joplin, Jasper County, Missouri and Picher, Ottawa County, Oklahoma
- From the Elmwood mine, near Carthage, Smith County, Tennessee
- the Eagle mine, Gilman district, Eagle County, Colorado
- In Mexico, from Santa Eulalia and Naica, Chihuahua, and Cananea, Sonora
- Huaron, Casapalca, and Huancavelica, Peru
- In Zinkgruvan, Sweden.
Gemstone use


Crystals of suitable size and transparency have been fashioned into gemstones, usually featuring the brilliant cut to best display sphalerite's high dispersion of 0.156 (B-G interval)—over three times that of diamond. Freshly cut gems have an adamantine luster. Owing to their softness and fragility the gems are often left unset as collector's or museum pieces (although some have been set into pendants). Gem-quality material is usually a yellowish to honey brown, red to orange, or green.
See also
References
- ↑ Sphalerite. Webmineral. Retrieved on 2011-06-20.
- 1 2 Sphalerite. Mindat.org. Retrieved on 2011-06-20.
- 1 2 Handbook of Mineralogy
- ↑ International Centre for Diffraction Data reference 04-004-3804 , ICCD reference 04-004-3804.
- Dana's Manual of Mineralogy ISBN 0-471-03288-3
- Webster, R., Read, P. G. (Ed.) (2000). Gems: Their sources, descriptions and identification (5th ed.), p. 386. Butterworth-Heinemann, Great Britain. ISBN 0-7506-1674-1
- Minerals.net
- Minerals of Franklin, NJ
External links
| Wikimedia Commons has media related to Sphalerite. |
- The sphalerite structure
- Possible relation of Sphalerite to origins of life and precursor chemicals in 'Primordial Soup'
| ||||||||||||||||||||||||
