Zinc–air battery
| Specific energy | (1.692, 4.932 MJ/kg) |
|---|---|
| Energy density | (5.328–35.21 MJ/L) |
| Specific power | 100 W/kg[3][4] |
| Nominal cell voltage | 1.65 V |

Zinc–air batteries (non-rechargeable; IEC codes: A, P), and zinc–air fuel cells (mechanically rechargeable) are metal-air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce. Sizes range from very small button cells for hearing aids, larger batteries used in film cameras that previously used mercury batteries, to very large batteries used for electric vehicle propulsion.
During discharge, a mass of zinc particles forms a porous anode, which is saturated with an electrolyte. Oxygen from the air reacts at the cathode and forms hydroxyl ions which migrate into the zinc paste and form zincate (Zn(OH)2−
4), releasing electrons to travel to the cathode. The zincate decays into zinc oxide and water returns to the electrolyte. The water and hydroxyl from the anode are recycled at the cathode, so the water is not consumed. The reactions produce a theoretical 1.65 volts, but this is reduced to 1.35–1.4 V in available cells.
Zinc–air batteries have some properties of fuel cells as well as batteries: the zinc is the fuel, the reaction rate can be controlled by varying the air flow, and oxidized zinc/electrolyte paste can be replaced with fresh paste.
Zinc-air batteries can be used to replace now discontinued 1.35 V mercury batteries (although with a significantly shorter operating life), which in the 1970s through 1980s were commonly used in photo cameras.
Possible future applications of this battery include its deployment as an electric vehicle battery and as a utility-scale energy storage system.
History
The effect of oxygen was known early in the 19th century when wet-cell Leclanche batteries absorbed atmospheric oxygen into the carbon cathode current collector. In 1878, a porous platinized carbon air electrode was found to work as well as the manganese dioxide (MnO
2) of the Leclanche cell. Commercial products began to be made on this principle in 1932 when George W. Heise and Erwin A. Schumacher of the National Carbon Company built cells,[5] treating the carbon electrodes with wax to prevent flooding. This type is still used for large zinc–air cells for navigation aids and rail transportation. However, the current capacity is low and the cells are bulky.
Large primary zinc–air cells such as the Thomas A. Edison Industries Carbonaire type were used for railway signaling, remote communication sites, and navigation buoys. These were long-duration, low-rate applications. Development in the 1970s of thin electrodes based on fuel-cell research allowed application to small button and prismatic primary cells for hearing aids, pagers, and medical devices, especially cardiac telemetry.[6]
The first rechargeable zinc air batteries were manufactured in 1996 by a Slovenian innovator Miro Zoric. They were developed to power vehicles using the first AC-based drive trains, also developed by Mr. Zoric. The first vehicles on roads to use zinc air batteries were small and mid-sized buses in Singapore, where Mr. Zoric led the national electrification program at Singapore Polytechnic, during his technology transfer post. The mass production assembly line for his zinc air batteries was put in place in 1997. The cells offered much higher energy density and specific energy (and weight) ratio, compared to then standard lead acid batteries.
Reaction formulas
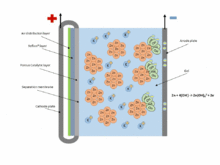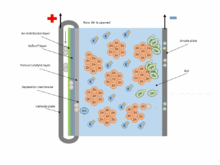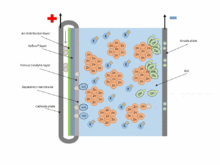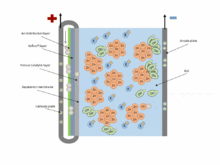
The chemical equations for the zinc–air cell are:[2]
- Anode: Zn + 4OH− → Zn(OH)42− + 2e− (E0 = -1.25 V)
- Fluid: Zn(OH)42− → ZnO + H2O + 2OH−
- Cathode: 1/2 O2 + H2O + 2e− → 2OH− (E0 = 0.34 V pH=11)
- Overall: 2Zn + O2 → 2ZnO (E0 = 1.59 V)
Zinc–air batteries cannot be used in a sealed battery holder since some air must come in; the oxygen in 1 liter of air is required for every ampere-hour of capacity used.
Storage density
Zinc-air batteries have higher energy density and specific energy (and weight) ratio than other types of battery because atmospheric air is one of the battery reactants. The air is not packaged with the battery, so that a cell can use more zinc in the anode than a cell that must also contain, for example, manganese dioxide. This increases capacity for a given weight or volume. As a specific example, a zinc–air battery of 11.6 mm diameter and height 5.4 mm from one manufacturer has a capacity of 620 mAh and weight 1.9 g; various silver oxide and alkaline cells of the same size supply 150–200 mAh and weigh 2.3–2.4 g.[7]
Storage and operating life
Zinc-air cells have long shelf life if sealed to keep air out; even miniature button cells can be stored for up to 3 years at room temperature with little capacity loss if their seal is not removed. Industrial cells stored in a dry state have an indefinite storage life.
The operating life of a zinc–air cell is a critical function of its interaction with its environment. The electrolyte loses water more rapidly in conditions of high temperature and low humidity. Because the potassium hydroxide electrolyte is deliquescent, in very humid conditions excess water accumulates in the cell, flooding the cathode and destroying its active properties. Potassium hydroxide also reacts with atmospheric carbon dioxide; carbonate formation eventually reduces electrolyte conductivity. Miniature cells have high self-discharge once opened to air; the cell's capacity is intended to be used within a few weeks.[6]
Discharge properties
Because the cathode does not change properties during discharge, terminal voltage is quite stable until the cell approaches exhaustion.
Power capacity is a function of several variables: cathode area, air availability, porosity, and the catalytic value of the cathode surface. Oxygen entry into the cell must be balanced against electrolyte water loss; cathode membranes are coated with (hydrophobic) Teflon material to limit water loss. Low humidity increases water loss; if enough water is lost the cell fails. Button cells have a limited current drain; for example an IEC PR44 cell has a capacity of 600 milliamp-hours (mAh) but a maximum current of only 22 milliamps (mA). Pulse load currents can be much higher since some oxygen remains in the cell between pulses.[6]
Low temperature reduces primary cell capacity but the effect is small for low drains. A cell may deliver 80% of its capacity if discharged over 300 hours at 0 °C (32 °F), but only 20% of capacity if discharged at a 50-hour rate at that temperature. Lower temperature also reduces cell voltage.
Cell types
Primary (non-rechargeable)
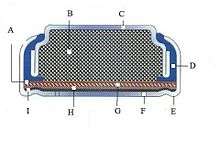
Large zinc–air batteries, with capacities up to 2,000 ampere–hours per cell, are used to power navigation instruments and marker lights, oceanographic experiments and railway signals.
Primary cells are made in button format to about 1 Ah. Prismatic shapes for portable devices are manufactured with capacities between 5 and 30 Ah. Hybrid cell cathodes include manganese dioxide to allow high peak currents.
Button cells are highly effective, but it is difficult to extend the same construction to larger sizes due to air diffusion performance, heat dissipation, and leakage problems. Prismatic and cylindrical cell designs address these problems. Stacking prismatic cells requires air channels in the battery and may require a fan to force air through the stack.[6]
Secondary (rechargeable)
Rechargeable zinc–air cells require zinc precipitation from the water-based electrolyte to be closely controlled. Challenges include dendrite formation, non-uniform zinc dissolution and limited solubility in electrolytes. Electrically reversing the reaction at a bi-functional air cathode, to liberate oxygen from discharge reaction products, is difficult; membranes tested to date have low overall efficiency. Charging voltage is much higher than discharge voltage, producing cycle energy efficiency as low as 50%. Providing charge and discharge functions by separate uni-functional cathodes, increases cell size, weight and complexity.[6] A satisfactory electrically recharged system potentially offers low material cost and high specific energy. As of 2014, only one company has commercial units for sale, as described in a Dept. of Energy produced video at the ARPA-e Energy Innovation Summit in 2013.[8] Fluidic Energy has apparently covered hundreds of thousands of outages in Asia[9] at distributed critical load sites. And at least one firm claims to be in field tests for grid-scale backup applications.[10]
Mechanical recharge
Rechargeable systems may mechanically replace the anode and electrolyte, essentially operating as a refurbishable primary cell, or may use zinc powder or other methods to replenish the reactants. Mechanically recharged systems were investigated for military electronics uses in the 1960s because of the high energy density and easy recharging. However, primary lithium batteries offered higher discharge rates and easier handling.
Mechanical recharging systems have been researched for decades for use in electric vehicles. Some approaches use a large zinc–air battery to maintain charge on a high discharge–rate battery used for peak loads during acceleration. Zinc granules serve as the reactant. Vehicles recharge via exchanging used electrolyte and depleted zinc for fresh reactants at a service station.
The term zinc–air fuel cell usually refers to a zinc–air battery in which zinc metal is added and zinc oxide is removed continuously. Zinc electrolyte paste or pellets are pushed into a chamber, and waste zinc oxide is pumped into a waste tank or bladder inside the fuel tank. Fresh zinc paste or pellets are taken from the fuel tank. The zinc oxide waste is pumped out at a refueling station for recycling. Alternatively, this term may refer to an electrochemical system in which zinc is a co-reactant assisting the reformation of hydrocarbons at the anode of a fuel cell.
Materials
Catalysts
Cobalt oxide/carbon nanotube hybrid oxygen reduction catalyst and Nickel-iron layered double hydroxide oxygen evolution cathode catalysts exhibited higher catalytic activity and durability in concentrated alkaline electrolytes than precious metal Platinum and Iridium catalysts. The resulting primary zinc-air battery showed peak power density of ~265 mW/cm3, current density of ~200 mA/cm3 at 1 V and energy density >700 Wh/kg.[11][12]
Rechargeable Zn-air batteries in a tri-electrode configuration exhibited an unprecedented small charge–discharge voltage polarization of ~0.70 V at 20 mA/cm3, high reversibility and stability over long charge and discharge cycles.[11][12]
In 2015, researchers announced a carbon-based, metal-free electrocatalyst that works efficiently in both reduction and oxygenation reactions. Organic compound aniline, polymerized into long chains in a phytic acid solution, was freeze-dried into a stable, mesoporous carbon aerogel with 2-50 nm pores, providing high surface area and room for the battery electrolyte to diffuse. The researchers pyrolized the aerogel to 1,000 degrees Celsius, turning the foam into a graphitic network, with many catalytic graphene edges. The aniline doped the foam with nitrogen, which enhances reduction. Phytic acid infuses the foam with phosphorus, helping oxygen evolution.[13] The foam has a surface area of ∼1,663 m2/gr. Primary batteries demonstrated an open-circuit potential of 1.48 V, a specific capacity of 735 mAh/gr (Zn) (energy density of 835 Wh/kg (Zn)), a peak power density of 55 mW/cm³ and stable operation for 240 h after mechanical recharging. Two-electrode rechargeable batteries cycled stably for 180 cycles at 2 mA/cm3.[14]
Applications
Vehicle propulsion
Metallic zinc could be used as an alternative fuel for vehicles, either in a zinc–air battery[15] or to generate hydrogen near the point of use. Zinc's characteristics have motivated considerable interest as an energy source for electric vehicles. Gulf General Atomic demonstrated a 20 kW vehicle battery. General Motors conducted tests in the 1970s. Neither project led to a commercial product.[16]
In addition to liquid, pellets could be formed that are small enough to pump. Fuel cells using pellets would be able to quickly replace zinc-oxide with fresh zinc metal.[17] The spent material can be recycled. The zinc–air cell is a primary cell (non-rechargeable); recycling is required to reclaim the zinc; much more energy is required to reclaim the zinc than is usable in a vehicle.
One advantage of utilizing zinc–air batteries for vehicle propulsion is that earth's supply of zinc metal is 100 times greater than that of lithium, per unit of battery energy. Current yearly global zinc production is sufficient to produce enough zinc-air batteries to power over one billion electric vehicles, whereas current lithium production is only sufficient to produce ten million lithium-ion powered vehicles.[18] Approximately 35% of the world's supply, or 1.8 gigatons of zinc reserves are in the United States,[19] whereas the U.S. holds only 0.38% of known lithium reserves.
Initial rechargeable zinc air batteries, developed for use in vehicles, were used for buses in Singapore. Their developer, Miro Zoric, chose zinc air chemistry specifically due to zinc air battery production requiring only abundant raw materials, which would, when used to power vehicular AC(induction) drive trains, which also do not require rare earth materials, allow global road transport electrification, without destabilizing global supply chains, or face and cause adverse raw material bottlenecks.
Grid storage
The Eos Energy System battery is about half the size of a shipping container and provides 1 MWh of storage. Con Edison, National Grid, Enel and GDF SUEZ began testing the battery for grid storage. Con Edison and City University of New York are testing a zinc-based battery from Urban Electric Power as part of a New York State Energy Research and Development Authority program. Eos projects that costs of $160 per kilowatt-hour and that it will provide electricity cheaper than a new natural-gas peaking power station. The cost of storing electricity with such EOS batteries is claimed to be U$160/kwh.[20] Other battery technologies range from $400 to about $1,000 a kilowatt-hour.[21]
Alternative configurations
Attempts to address zinc–air's limitations include:[22]
- Pumping zinc slurry through the battery in one direction for charging and reversing for discharge. Capacity is limited only by the slurry reservoir size.
- Alternate electrode shapes (via gelling and binding agents)
- Humidity management
- Careful catalyst dispersal to improve oxygen reduction and production
- Modularizing components for repair without complete replacement
Safety and environment
Zinc corrosion can produce potentially explosive hydrogen. Vent holes prevent pressure build-up within the cell. Manufacturers caution against hydrogen build-up in enclosed areas. A short-circuited cell gives relatively low current. Deep discharge below 0.5 V/cell may result in electrolyte leakage; little useful capacity exists below 0.9 V/cell.
Older designs used mercury amalgam amounting to about 1% of the weight of a button cell, to prevent zinc corrosion. Newer types have no added mercury. Zinc itself is relatively low in toxicity. Mercury-free designs require no special handling when discarded or recycled.[6]
In United States waters, environmental regulations now require proper disposal of primary batteries removed from navigation aids. Formerly, discarded zinc–air primary batteries were dropped into the water around buoys, which allowed mercury to escape into the environment.[23]
See also
- Aluminium–air battery
- Fluidic Energy
- Fuel cell
- Gas diffusion electrode
- Hydrogen technologies
- Metal–air electrochemical cell
- Zinc-bromide battery
References
- ↑ power one: Hearing Aid Batteries. Powerone-batteries.com. Retrieved on 2012-09-30.
- 1 2 Duracell: Zinc–air Technical Bulletin. duracell.com
- ↑ zincair_hybrid. greencarcongress (2004-11-03). Retrieved on 2012-09-30.
- ↑ battery types. thermoanalytics. Retrieved on 2012-09-30.
- ↑ US 1899615 Air-depolarized primary battery Heise – February, 1933
- 1 2 3 4 5 6 David Linden, Thomas B. Reddy (ed). Handbook Of Batteries 3rd Edition, McGraw-Hill, New York, 2002 ISBN 0-07-135978-8, chapter 13 and chapter 38
- ↑ "Energizer Technical Information". Data.energizer.com. 2004-01-01. Retrieved 2013-06-01.
- ↑ http://vimeo.com/60446135
- ↑ http://www.fluidicenergy.com
- ↑ "Eos Puts Its Zinc-Air Grid Batteries to the Test With ConEd". Greentech Media. 2013-05-02. Retrieved 2013-10-08.
- 1 2 Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J. E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. (2013). "Advanced zinc-air batteries based on high-performance hybrid electrocatalysts". Nature Communications 4: 1805. doi:10.1038/ncomms2812. PMID 23651993.
- 1 2 First Posted: May 29, 2013 06:22 PM EDT. "New High-Efficiency Zinc-Air Batteries Much Cheaper Than Lithium-Ion : Tech". Science World Report. Retrieved 2013-06-01.
- ↑ Mayhood, Kevin (2015-04-06). "Researchers create first metal-free catalyst for rechargeable zinc-air batteries". R&D.
- ↑ Template:Nature Nanotechnology
- ↑ J. Noring et al, Mechanically refuelable zinc–air electric vehicle cells in Proceedings of the Symposium on Batteries and Fuel Cells for Stationary and Electric Vehicle Applications Volumes 93–98 of Proceedings (Electrochemical Society), The Electrochemical Society, 1993 ISBN 1-56677-055-6 pp. 235–236
- ↑ C. A. C. Sequeira Environmental oriented electrochemistry Elsevier, 1994 ISBN 0-444-89456-X, pp. 216–217
- ↑ "Science & Technology Review". Llnl.gov. 1995-10-16. Retrieved 2013-10-08.
- ↑ William Tahil (December 2006). The Trouble with Lithium Implications of Future PHEV Production for Lithium Demand. Meridian International Research
- ↑ Zinc air fuel cell provides more benefits than lithium ion batteries. Machine Design (2010-10-07). Retrieved on 2012-09-30.
- ↑
- ↑
- ↑ Bullis, Kevin (October 28, 2009). "High-Energy Batteries Coming to Market". Technology Review. Retrieved June 15, 2010.
- ↑ U.S.C.G. Directive, retrieved 2010 Jan 18.
External links
- Zinc–air powered buses
- Military uses of Zinc–air Batteries
- Zinc-Air Batteries for UAVs and MAVs
- Incorrect zinc–air reaction
- Zinc–air fuel cell
- Procedure to make a simple zinc–air fuel cell as a science fair project.
- ReVolt Technology developing rechargeable zinc–air batteries
- Duracell technical bulletin (suppliers of zinc–air hearing aid batteries)
- Overview of batteries
- Revolt Introduction
- Metal Air Batteries
Further reading
- Heise, G. W. and Schumacher, E. A., An Air-Depolarized Primary Cell with Caustic Alkali Electrolyte, Transactions of the Electrochemical Society, Vol. 62, Page 363, 1932.
| ||||||||||||||||||||
| ||||||||||||||||||||||
