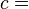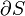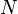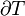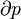Zeroth law of thermodynamics
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
Two systems are said to be in the relation of thermal equilibrium if they are linked by a wall permeable only to heat, and do not change over time.[1] As a convenience of language, systems are sometimes also said to be in a relation of thermal equilibrium if they are not linked so as to be able to transfer heat to each other, but would not do so if they were connected by a wall permeable only to heat. Thermal equilibrium between two systems is a transitive relation.
The physical meaning of the law was expressed by Maxwell in the words: "All heat is of the same kind".[2] For this reason, another statement of the law is "All diathermal walls are equivalent".[3]
The law is important for the mathematical formulation of thermodynamics, which needs the assertion that the relation of thermal equilibrium is an equivalence relation. This information is needed for a mathematical definition of temperature that will agree with the physical existence of valid thermometers.[4]
Zeroth law as equivalence relation
A thermodynamic system is by definition in its own state of internal thermodynamic equilibrium, that is to say, there is no change in its observable state (i.e. macrostate) over time and no flows occur in it. One precise statement of the zeroth law is that the relation of thermal equilibrium is an equivalence relation on pairs of thermodynamic systems.[5] In other words, the set of all systems each in its own state of internal thermodynamic equilibrium may be divided into subsets in which every system belongs to one and only one subset, and is in thermal equilibrium with every other member of that subset, and is not in thermal equilibrium with a member of any other subset. This means that a unique "tag" can be assigned to every system, and if the "tags" of two systems are the same, they are in thermal equilibrium with each other, and if different, they are not. This property is used to justify the use of empirical temperature as a tagging system. Empirical temperature provides further relations of thermally equilibrated systems, such as order and continuity with regard to "hotness" or "coldness", but these are not implied by the standard statement of the zeroth law.
If it is defined that a thermodynamic system is in thermal equilibrium with itself (i.e., thermal equilibrium is reflexive), then the zeroth law may be stated as follows:[6]
If a body A, be in thermal equilibrium with two other bodies, B and C, then B and C are in thermal equilibrium with one another.
This statement asserts that thermal equilibrium is a left-Euclidean relation between thermodynamic systems. If we also define that every thermodynamic system is in thermal equilibrium with itself, then thermal equilibrium is also a reflexive relation. Binary relations that are both reflexive and Euclidean are equivalence relations. Thus, again implicitly assuming reflexivity, the zeroth law is therefore often expressed as a right-Euclidean statement:[7]
If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
One consequence of an equivalence relationship is that the equilibrium relationship is symmetric: If A is in thermal equilibrium with B, then B is in thermal equilibrium with A. Thus we may say that two systems are in thermal equilibrium with each other, or that they are in mutual equilibrium. Another consequence of equivalence is that thermal equilibrium is a transitive relationship and is occasionally expressed as such:[4][8]
If A is in thermal equilibrium with B and if B is in thermal equilibrium with C, then A is in thermal equilibrium with C .
A reflexive, transitive relationship does not guarantee an equivalence relationship. In order for the above statement to be true, both reflexivity and symmetry must be implicitly assumed.
It is the Euclidean relationships which apply directly to thermometry. An ideal thermometer is a thermometer which does not measurably change the state of the system it is measuring. Assuming that the unchanging reading of an ideal thermometer is a valid "tagging" system for the equivalence classes of a set of equilibrated thermodynamic systems, then if a thermometer gives the same reading for two systems, those two systems are in thermal equilibrium, and if we thermally connect the two systems, there will be no subsequent change in the state of either one. If the readings are different, then thermally connecting the two systems will cause a change in the states of both systems and when the change is complete, they will both yield the same thermometer reading. The zeroth law provides no information regarding this final reading.
Foundation of temperature
The zeroth law establishes thermal equilibrium as an equivalence relationship. An equivalence relationship on a set (such as the set of all systems each in its own state of internal thermodynamic equilibrium) divides that set into a collection of distinct subsets ("disjoint subsets") where any member of the set is a member of one and only one such subset. In the case of the zeroth law, these subsets consist of systems which are in mutual equilibrium. This partitioning allows any member of the subset to be uniquely "tagged" with a label identifying the subset to which it belongs. Although the labeling may be quite arbitrary,[9] temperature is just such a labeling process which uses the real number system for tagging. The zeroth law justifies the use of suitable thermodynamic systems as thermometers to provide such a labeling, which yield any number of possible empirical temperature scales, and justifies the use of the second law of thermodynamics to provide an absolute, or thermodynamic temperature scale. Such temperature scales bring additional continuity and ordering (i.e., "hot" and "cold") properties to the concept of temperature.[7]
In the space of thermodynamic parameters, zones of constant temperature form a surface, that provides a natural order of nearby surfaces. One may therefore construct a global temperature function that provides a continuous ordering of states. The dimensionality of a surface of constant temperature is one less than the number of thermodynamic parameters, thus, for an ideal gas described with three thermodynamic parameters P, V and n, it is a two-dimensional surface.
For example, if two systems of ideal gases are in equilibrium, then P1V1/N1 = P2V2/N2 where Pi is the pressure in the ith system, Vi is the volume, and Ni is the amount (in moles, or simply the number of atoms) of gas.
The surface PV/N = const defines surfaces of equal thermodynamic temperature, and one may label defining T so that PV/N = RT, where R is some constant. These systems can now be used as a thermometer to calibrate other systems. Such systems are known as "ideal gas thermometers".
In a sense, focused on in the zeroth law, there is only one kind of diathermal wall or one kind of heat, as expressed by Maxwell's dictum that "All heat is of the same kind".[2] But in another sense, heat is transferred in different ranks, as expressed by Sommerfeld's dictum "Thermodynamics investigates the conditions that govern the transformation of heat into work. It teaches us to recognize temperature as the measure of the work-value of heat. Heat of higher temperature is richer, is capable of doing more work. Work may be regarded as heat of an infinitely high temperature, as unconditionally available heat."[10] This is why temperature is the particular variable indicated by the zeroth law's statement of equivalence.
Physical meaning of the usual statement of the zeroth law
The present article states the zeroth law as it is often summarized in textbooks. Nevertheless, this usual statement perhaps does not explicitly convey the full physical meaning that underlies it. The underlying physical meaning was perhaps first clarified by Maxwell in his 1871 textbook.[2]
In Carathéodory's (1909) theory, it is postulated that there exist walls "permeable only to heat", though heat is not explicitly defined in that paper. This postulate is a physical postulate of existence. It does not, however, as worded just previously, say that there is only one kind of heat. This paper of Carathéodory states as proviso 4 of its account of such walls: "Whenever each of the systems S1 and S2 is made to reach equilibrium with a third system S3 under identical conditions, systems S1 and S2 are in mutual equilibrium".[11] It is the function of this statement in the paper, not there labeled as the zeroth law, to provide not only for the existence of transfer of energy other than by work or transfer of matter, but further to provide that such transfer is unique in the sense that there is only one kind of such wall, and one kind of such transfer. This is signaled in the postulate of this paper of Carathéodory that precisely one non-deformation variable is needed to complete the specification of a thermodynamic state, beyond the necessary deformation variables, which are not restricted in number. It is therefore not exactly clear what Carathéodory means when in the introduction of this paper he writes "It is possible to develop the whole theory without assuming the existence of heat, that is of a quantity that is of a different nature from the normal mechanical quantities."
Maxwell (1871) discusses at some length ideas which he summarizes by the words "All heat is of the same kind".[2] Modern theorists sometimes express this idea by postulating the existence of a unique one-dimensional hotness manifold, into which every proper temperature scale has a monotonic mapping.[12] This may be expressed by the statement that there is only one kind of temperature, regardless of the variety of scales in which it is expressed. Another modern expression of this idea is that "All diathermal walls are equivalent".[13] This might also be expressed by saying that there is precisely one kind of non-mechanical, non-matter-transferring contact equilibrium between thermodynamic systems.
These ideas may be regarded as helping to clarify the physical meaning of the usual statement of the zeroth law of thermodynamics. It is the opinion of Lieb and Yngvason (1999) that the derivation from statistical mechanics of the law of entropy increase is a goal that has so far eluded the deepest thinkers.[14] Thus the idea remains open to consideration that the existence of heat and temperature are needed as coherent primitive concepts for thermodynamics, as expressed, for example, by Maxwell and Planck. On the other hand, Planck in 1926 clarified how the second law can be stated without reference to heat or temperature, by referring to the irreversible and universal nature of friction in natural thermodynamic processes.[15]
History
According to Arnold Sommerfeld, Ralph H. Fowler invented the title 'the zeroth law of thermodynamics' when he was discussing the 1935 text of Saha and Srivastava. They write on page 1 that "every physical quantity must be measurable in numerical terms". They presume that temperature is a physical quantity and then deduce the statement "If a body A is in temperature equilibrium with two bodies B and C, then B and C themselves will be in temperature equilibrium with each other". They then in a self-standing paragraph italicize as if to state their basic postulate: "Any of the physical properties of A which change with the application of heat may be observed and utilised for the measurement of temperature." They do not themselves here use the term 'zeroth law of thermodynamics'.[16][17] There are very many statements of these physical ideas in the physics literature long before this text, in very similar language. What was new here was just the label 'zeroth law of thermodynamics'. Fowler, with co-author Edward A. Guggenheim, wrote of the zeroth law as follows:
- ...we introduce the postulate: If two assemblies are each in thermal equilibrium with a third assembly, they are in thermal equilibrium with each other.
They then proposed that "it may be shown to follow that the condition for thermal equilibrium between several assemblies is the equality of a certain single-valued function of the thermodynamic states of the assemblies, which may be called the temperature t, any one of the assemblies being used as a "thermometer" reading the temperature t on a suitable scale. This postulate of the "Existence of temperature" could with advantage be known as the zeroth law of thermodynamics". The first sentence of this present article is a version of this statement.[18] It is not explicitly evident in the existence statement of Fowler and Guggenheim that temperature refers to a unique attribute of a state of a system, such as is expressed in the idea of the hotness manifold. Also their statement refers explicitly to statistical mechanical assemblies, not explicitly to macroscopic thermodynamically defined systems.
References
Citations
- ↑ Carathéodory, C. (1909).
- 1 2 3 4 Maxwell, J.C. (1871), p. 57.
- ↑ Bailyn, M. (1994), pp. 24, 144.
- 1 2 Lieb, E.H., Yngvason, J. (1999), p. 56.
- ↑ Lieb, E.H., Yngvason, J. (1999), p. 52.
- ↑ Planck. M. (1914), p. 2.
- 1 2 Buchdahl, H.A. (1966), p. 73.
- ↑ Kondepudi, D. (2008), p. 7.
- ↑ Dugdale, J.S. (1996), p. 35.
- ↑ Sommerfeld, A. (1923), p. 36.
- ↑ Carathéodory, C. (1909), Section 6.
- ↑ Serrin, J. (1986), p. 6.
- ↑ Bailyn, M. (1994), p. 23.
- ↑ Lieb, E.H., Yngvason, J. (1999), p. 5.
- ↑ Planck, M. (1926).
- ↑ Sommerfeld, A. (1951/1955), p. 1.
- ↑ Saha, M.N., Srivastava, B.N. (1935), p. 1.
- ↑ Fowler, R., Guggenheim, E.A. (1939/1965), p. 56.
Works cited
- Bailyn, M. (1994). A Survey of Thermodynamics, American Institute of Physics Press, New York, ISBN 978-0-88318-797-5.
- H.A. Buchdahl (1966). The Concepts of Classical Thermodynamics. Cambridge University Press.
- C. Carathéodory (1909). "Untersuchungen über die Grundlagen der Thermodynamik". Mathematische Annalen (in German) 67: 355–386. doi:10.1007/BF01450409. A translation may be found here. A partly reliable translation is to be found at Kestin, J. (1976). The Second Law of Thermodynamics, Dowden, Hutchinson & Ross, Stroudsburg PA.
- Dugdale, J. S. (1996). Entropy and its Physical Interpretation. Taylor & Francis. ISBN 0-7484-0569-0.
- Fowler, R., Guggenheim, E.A. (1939/1965). Statistical Thermodynamics. A version of Statistical Mechanics for Students of Physics and Chemistry, first printing 1939, reprinted with corrections 1965, Cambridge University Press, Cambridge UK.
- D. Kondepudi (2008). Introduction to Modern Thermodynamics. Wiley. ISBN 978-0470-01598-8.
- Lieb, E.H., Yngvason, J. (1999). The physics and mathematics of the second law of thermodynamics, Physics Reports, 310: 1–96.
- Maxwell, J.C. (1871). Theory of Heat, Longmans, Green, and Co., London.
- Planck. M. (1914). The Theory of Heat Radiation, a translation by Masius, M. of the second German edition, P. Blakiston's Son & Co., Philadelphia.
- Planck, M. (1926). Über die Begründing des zweiten Hauptsatzes der Thermodynamik, S.B. Preuß. Akad. Wiss. phys. math. Kl.: 453–463.
- Saha, M.N., Srivastava, B.N. (1935). A Treatise on Heat. (Including Kinetic Theory of Gases, Thermodynamics and Recent Advances in Statistical Thermodynamics), the second and revised edition of A Text Book of Heat, The Indian Press, Allahabad and Calcutta.
- Serrin, J. (1986). Chapter 1, 'An Outline of Thermodynamical Structure', pages 3–32, in New Perspectives in Thermodynamics, edited by J. Serrin, Springer, Berlin, ISBN 3-540-15931-2.
- Sommerfeld, A. (1923). Atomic Structure and Spectral Lines, translated from the third German edition by H.L. Brose, Methuen, London.
- Sommerfeld, A. (1951/1955). Thermodynamics and Statistical Mechanics, vol. 5 of Lectures on Theoretical Physics, edited by F. Bopp, J. Meixner, translated by J. Kestin, Academic Press, New York.
Further reading
- Atkins, Peter (2007). Four Laws That Drive the Universe. New York: Oxford University Press. ISBN 978-0-19-923236-9.