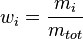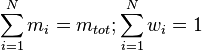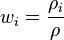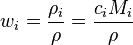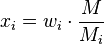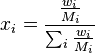Mass fraction (chemistry)
In chemistry, the mass fraction  is the ratio of one substance with mass
is the ratio of one substance with mass 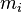 to the mass of the total mixture
to the mass of the total mixture 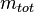 , defined as[1]
, defined as[1]
The sum of all the mass fractions is equal to 1:
Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (frequently, though erroneously, called percentage by weight, abbreviated wt%). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction (percentage by volume, vol%) are others.
For elemental analysis, mass fraction (or "mass percent composition") can also refer to the ratio of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula[2] or its chemical formula[3]
Terminology
"Percent concentration" does not refer to this quantity. This improper name persists, especially in elementary textbooks. In biology, the unit "%" is sometimes (incorrectly) used to denote mass concentration, also called "mass/volume percentage." A solution with 1 g of solute dissolved in a final volume of 100 mL of solution would be labeled as "1 %" or "1 % m/v" (mass/volume). This is incorrect because the unit "%" can only be used for dimensionless quantities. Instead, the concentration should simply be given in units of g/mL. "Percent solution" or "percentage solution" are thus terms best reserved for "mass percent solutions" (m/m = m% = mass solute/mass total solution after mixing), or "volume percent solutions" (v/v = v% = volume solute per volume of total solution after mixing). The very ambiguous terms "percent solution" and "percentage solutions" with no other qualifiers continue to occasionally be encountered.
In thermal engineering vapor quality is used for the mass fraction of vapor in the steam.
In alloys, especially those of noble metals, the term fineness is used for the mass fraction of the noble metal in the alloy.
Properties
The mass fraction is independent of temperature.
Related quantities
Mass concentration
The mass fraction of a component in a solution is the ratio of the mass concentration of that component  (density of that component in the mixture) to the density of solution
(density of that component in the mixture) to the density of solution  .
.
Molar concentration
The relation to molar concentration is like that from above substituting the relation between mass and molar concentration.
Mass percentage
Multiplying mass fraction by 100 gives the mass percentage. It is sometimes called weight percent (wt%) or weight-weight percentage.
Mole fraction
The mole fraction  can be calculated using the formula
can be calculated using the formula
where  is the molar mass of the component
is the molar mass of the component  and
and 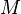 is the average molar mass of the mixture.
is the average molar mass of the mixture.
Replacing the expression of the molar mass-produces:
Spatial variation and gradient
In a spatially non-uniform mixture, the mass fraction gradient triggers the phenomenon of diffusion.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "mass fraction".
- ↑ Formula from Mass Composition
- ↑
| ||||||||||||||||||