Herring

Herring are forage fish, mostly belonging to the family Clupeidae.
Herring often move in large schools around fishing banks and near the coast. The most abundant and commercially important species belong to the genus Clupea, found particularly in shallow, temperate waters of the North Pacific and the North Atlantic oceans, including the Baltic Sea, as well as off the west coast of South America. Three species of Clupea are recognised, and provide about 90% of all herrings captured in fisheries. Most abundant of all is the Atlantic herring, providing over half of all herring capture. Fishes called herring are also found in India, in the Arabian Sea, Indian Ocean and Bay of Bengal.
Herring played a pivotal role in the history of marine fisheries in Europe,[2] and early in the twentieth century their study was fundamental to the evolution of fisheries science.[3][4] These oily fish[5] also have a long history as an important food fish, and are often salted, smoked, or pickled.
Species
| This article is one of a series on |
| Commercial fish |
|---|
| |
| Large pelagic |
| billfish, bonito mackerel, salmon shark, tuna |
|
|
| Forage |
| anchovy, herring menhaden, sardine shad, sprat |
|
|
| Demersal |
| cod, eel, flatfish pollock, ray |
| Mixed |
| carp, tilapia |
A number of different species, most belonging to the family Clupeidae, are commonly referred to as herrings. The origins of the term herring is somewhat unclear, though it may derive from the Old High German heri meaning a "host, multitude", in reference to the large schools they form.[6]
The type genus of the herring family Clupeidae is Clupea.[4] Clupea contains three species: the Atlantic herring (the type species) found in the north Atlantic, the Pacific herring found in the north Pacific, and the Araucanian herring found off the coast of Chile. Subspecific divisions have been suggested for both the Atlantic and Pacific herrings, but their biological basis remain unclear.
| Herrings in the genus Clupea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Maximum length |
Common length |
Maximum weight |
Maximum age |
Trophic level |
Fish Base |
FAO | ITIS | IUCN status |
| Araucanian herring | Clupea bentincki Norman, 1936 | 28.4 cm | cm | kg | years | 2.69 | [7] | [8] | [9] | Not assessed |
| Atlantic herring | Clupea harengus Linnaeus, 1758 | 45.0 cm | 30.0 cm | 1.05 kg | 22 years | 3.23 | [10] | [11] | [12] | |
| Pacific herring | Clupea pallasii Valenciennes, 1847 | 46.0 cm | 25.0 cm | 19 years | 3.15 | [10] | [14] | [15] | Not assessed | |
In addition, a number of related species, all in the family Clupeidae, are commonly referred to as herrings. The table immediately below includes those members of the Clupeidae family referred to by FishBase as herrings which have been assessed by the International Union for Conservation of Nature.
| Other herrings in the family Clupeidae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Common name | Scientific name | Maximum length |
Common length |
Maximum weight |
Maximum age |
Trophic level |
Fish Base |
FAO | ITIS | IUCN status |
| Freshwater herrings | Toothed river herring | Clupeoides papuensis (Ramsay & Ogilby, 1886) | cm | cm | kg | years | [16] | [17] | | ||
| Round herrings | Day's round herring | Dayella malabarica (Day, 1873) | cm | cm | kg | years | [19] | [20] | | ||
| Dwarf round herring | Jenkinsia lamprotaenia (Gosse, 1851) | cm | cm | kg | years | [22] | [23] | | |||
| Gilchrist's round herring | Gilchristella aestuaria (Gilchrist, 1913 | cm | cm | kg | years | [25] | [26] | | |||
| Little-eye round herring | Jenkinsia majua Whitehead, 1963 | cm | cm | kg | years | [28] | [29] | | |||
| Red-eye round herring | Etrumeus teres (De Kay, 1842) | 33 cm | 25 cm | kg | years | [31] | [32] | [33] | Not assessed | ||
| Two-finned round herring | Spratellomorpha bianalis (Bertin, 1940) | 4.5 cm | cm | kg | years | 3.11 | [34] | [35] | | ||
| Whitehead's round herring | Etrumeus whiteheadi (Wongratana, 1983) | 20 cm | cm | kg | years | 3.4 | [37] | [38] | [39] | | |
| Venezuelan herring | Jenkinsia parvula Cervigón and Velasquez, 1978 | cm | cm | kg | years | [41] | [42] | | |||
| Thread herrings | Galapagos thread herring | Opisthonema berlangai (Günther, 1867) | 26 cm | 18 cm | kg | years | 3.27 | [44] | [45] | | |
| Middling thread herring | Opisthonema medirastre Berry & Barrett, 1963 | cm | cm | kg | years | [47] | [48] | | |||
| Pacific thread herring | Opisthonema libertate (Günther, 1867) | 30 cm | 22 cm | kg | years | [50] | [51] | [45] | | ||
| Slender thread herring | Opisthonema bulleri (Regan, 1904) | cm | cm | kg | years | [53] | [54] | | |||
| Other | Blackstripe herring | Lile nigrofasciata Castro-Aguirre Ruiz-Campos and Balart, 2002 | cm | cm | kg | years | [56] | [57] | | ||
| Denticle herring | Denticeps clupeoides Clausen, 1959 | cm | cm | kg | years | [59] | [60] | | |||
| Dogtooth herring | Chirocentrodon bleekerianus (Poey, 1867) | cm | cm | kg | years | [62] | [63] | | |||
| Graceful herring | Lile gracilis Castro-Aguirre and Vivero, 1990 | cm | cm | kg | years | [65] | [66] | | |||
| Pacific Flatiron herring | Harengula thrissina (Jordan and Gilbert, 1882) | cm | cm | kg | years | [68] | [69] | | |||
| Sanaga pygmy herring | Thrattidion noctivagus Roberts, 1972 | cm | cm | kg | years | [71] | [72] | | |||
| Silver-stripe round herring | Spratelloides gracilis (Temminck & Schlegel, 1846) | 10.5 cm | cm | kg | years | 3.0 | [74] | [75] | Not assessed | ||
| Striped herring | Lile stolifera (Jordan & Gilbert, 1882) | cm | cm | kg | years | [76] | [77] | | |||
| West African pygmy herring | Sierrathrissa leonensis Thys van den Audenaerde, 1969 | cm | cm | kg | years | [79] | [80] | | |||
There are also a number of other species called herrings, which may be related to clupeids or just share some characteristics of herrings (such as the lake herring, which is a salmonid). Just which of these species are called herrings can vary with locality, so what might be called a herring in one locality might be called something else in another locality. Some examples:
| Other fishes called herring | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Maximum length |
Common length |
Maximum weight |
Maximum age |
Trophic level |
Fish Base |
FAO | ITIS | IUCN status | |
| Longfin herring | Bigeyed longfin herring | Opisthopterus macrops (Günther, 1867) | cm | cm | kg | years | [82] | [83] | | ||
| Dove's longfin herring | Opisthopterus dovii (Günther 1868) | cm | cm | kg | years | [85] | [86] | | |||
| Hatchet herring | Ilisha fuerthii (Steindachner, 1875) | cm | cm | kg | years | [88] | [89] | | |||
| Panama longfin herring | Odontognathus panamensis (Steindachner, 1876) | cm | cm | kg | years | [91] | [92] | | |||
| Tropical longfin herring | Neoopisthopterus tropicus (Hildebrand 1946) | cm | cm | kg | years | [94] | [95] | | |||
| Vaqueira longfin herring | Opisthopterus effulgens (Regan 1903) | cm | cm | kg | years | [97] | [98] | | |||
| Equatorial longfin herring | Opisthopterus equatorialis Hildebrand, 1946 | cm | cm | kg | years | [100] | [101] | | |||
| Wolf herring | Dorab wolf-herring | Chirocentrus dorab (Forsskål, 1775) | 100 cm | 60 cm | kg | years | 4.50 | [103] | [104] | [105] | Not assessed |
| Whitefin wolf-herring | Chirocentrus nudus Swainson, 1839 | 100 cm | cm | 0.41 kg | years | 4.19 | [106] | [107] | Not assessed | ||
| Freshwater whitefish | Lake herring (cisco) | Coregonus artedi Lesueur, 1818 | cm | cm | kg | years | [108] | [109] | Not assessed | ||
Characteristics

The species of Clupea belong to the larger family Clupeidae (herrings, shads, sardines, menhadens), which comprises some 200 species that share similar features. These silvery-coloured fish have a single dorsal fin, which is soft, without spines. They have no lateral line and have a protruding lower jaw. Their size varies between subspecies: the Baltic herring (Clupea harengus membras) is small, 14 to 18 centimetres; the proper Atlantic herring (C. h. harengus) can grow to about 46 cm (18 inches) and weigh up 700 g (1.5 pounds); and Pacific herring grow to about 38 cm (15 inches).
Life cycle

At least one stock of Atlantic herring spawns in every month of the year. Each spawns at a different time and place (spring, summer, autumn and winter herrings). Greenland populations spawn in 0–5 metres (0–16 ft) of water while North Sea (bank) herrings spawn at up to 200 metres (660 ft) in autumn. Eggs are laid on the sea bed, on rock, stones, gravel, sand or beds of algae. "...the fish were darting rapidly about, and those who have opportunity to see the fish spawning in more shallow water ... state that both males and females are in constant motion, rubbing against one another and upon the bottom, apparently by pressure aiding in the discharge of the eggs and milt" (Moore at Cross Island, Maine).
Females may deposit from 20,000 up to 40,000 eggs, according to age and size, averaging about 30,000. In sexually mature herrings, the genital organs grow before spawning, reaching about one-fifth of its total weight.
The eggs sink to the bottom, where they stick in layers or clumps to gravel, seaweed or stones, by means of their mucus coating, or to any other objects on which they chance to settle.
If the egg layers are too thick they suffer from oxygen depletion and often die, entangled in a maze of fucus. They need substantial water microturbulence, generally provided by wave action or coastal currents. Survival is highest in crevices and behind solid structures, because predators feast on openly disposed eggs. The individual eggs are 1 to 1.4 millimetres (0.039 to 0.055 in) in diameter, depending on the size of the parent fish and also on the local race. Incubation time is about 40 days at 3 °C (37 °F), 15 days at 7 °C (45 °F), 11 days at 10 °C (50 °F). Eggs die at temperatures above 19 °C (66 °F).
The larvae are 5 to 6 millimetres (0.20 to 0.24 in) long at hatching, with a small yolk sac that is absorbed by the time the larva reaches 10 millimetres (0.39 in). Only the eyes are well pigmented. The rest of the body is nearly transparent, virtually invisible under water and in natural lighting conditions.
The dorsal fin forms at 15 to 17 millimetres (0.59 to 0.67 in), the anal fin at about 30 millimetres (1.2 in)—the ventral fins are visible and the tail becomes well forked at 30 to 35 millimetres (1.4 in)— at about 40 millimetres (1.6 in) the larva begins to look like a herring.
The larvae are very slender and can easily be distinguished from all other young fish of their range by the location of the vent, which lies close to the base of the tail. But distinguishing clupeoids one from another in their early stages requires critical examination, especially telling herring from sprats.
At one year they are about 10 centimetres (3.9 in) long, and they first spawn at three years.



Ecology
Prey
Herrings are a prominent converter of zooplankton into fish, consuming copepods, arrow worms, pelagic amphipods, mysids and krill in the pelagic zone. Conversely, they are a central prey item or forage fish for higher trophic levels. The reasons for this success is still enigmatic; one speculation attributes their dominance to the huge, extremely fast cruising schools they inhabit.
Young herring feed on phytoplankton and as they mature they start to consume larger organisms. Adult herring feed on zooplankton, tiny animals that are found in oceanic surface waters, and small fish and fish larvae. Copepods and other tiny crustaceans are the most common zooplankton eaten by herring. During daylight herring stay in the safety of deep water, feeding at the surface only at night when there is less chance of being seen by predators. They swim along with their mouths open, filtering the plankton from the water as it passes through their gills. Young herring mostly hunt copepods individually, by means of "particulate feeding" or "raptorial feeding",[110] a feeding method also used by adult herring on larger prey items like krill. If prey concentrations reach very high levels, as in microlayers, at fronts or directly below the surface, herring become filter feeders, driving several meters forward with wide open mouth and far expanded opercula, then closing and cleaning the gill rakers for a few milliseconds.
Copepods, the primary zooplankton, are a major item on the forage fish menu. Copepods are typically one millimetre (0.04 in) to two millimetres (0.08 in) long, with a teardrop shaped body. Some scientists say they form the largest animal biomass on the planet.[111] Copepods are very alert and evasive. They have large antennae (see photo below left). When they spread their antennae they can sense the pressure wave from an approaching fish and jump with great speed over a few centimetres. If copepod concentrations reach high levels, schooling herrings adopt a method called ram feeding. In the photo below, herring ram feed on a school of copepods. They swim with their mouth wide open and their opercula fully expanded.



The fish swim in a grid where the distance between them is the same as the jump length of their prey, as indicated in the animation above right. In the animation, juvenile herring hunt the copepods in this synchronised way. The copepods sense with their antennae the pressure-wave of an approaching herring and react with a fast escape jump. The length of the jump is fairly constant. The fish align themselves in a grid with this characteristic jump length. A copepod can dart about 80 times before it tires. After a jump, it takes it 60 milliseconds to spread its antennae again, and this time delay becomes its undoing, as the almost endless stream of herrings allows a herring to eventually snap the copepod. A single juvenile herring could never catch a large copepod.[110]
Other pelagic prey eaten by herrings includes fish eggs, larval snails, diatoms by herring larvae below 20 millimetres (0.79 in), tintinnids by larvae below 45 millimetres (1.8 in), molluscan larvae, menhaden larvae, krill, mysids, smaller fishes, pteropods, annelids, Calanus, Centropagidae and Meganyctiphanes norvegica.
Herrings, along with cod and sprat, are the most important commercial species to humans in the Baltic Sea.[112] The analysis of the stomach contents of these fish indicate cod is the top predator, preying on the herring and sprat.[112][113] Sprat are competitive with herrings for the same food resources. This is evident in the two species' vertical migration in the Baltic Sea, where they compete for the limited zooplankton that is available and necessary for their survival.[114] Sprat are highly selective in their diet and eat only zooplankton, while herrings are more eclectic, adjusting their diet as they grow in size.[114] In the Baltic, copepods of the genus Acartia can be present in large numbers. However, they are small in size with a high escape response, so herring and sprat avoid trying to catch them. These copepods also tend to dwell more in surface waters, whereas herrings and sprat, especially during the day, tend to dwell in deeper waters.[114]
Predators


- See also: Predator avoidance in schooling fish, Bait ball
Predators of herring include seabirds, marine mammals such as dolphins, porpoises, whales, seals and sea lions, predatory fish such as sharks, billfish, tuna, salmon, striped bass, cod and halibut, and fishermen.
The predators often operate cooperatively in groups, using different techniques to panic or herd a school of herrings into a tight bait ball. Different predators species then use different techniques to pick the fish off in the bait ball. The sailfish raises its sail to make it appear much larger. Swordfish charge at high speed through the bait balls, slashing with their swords to kill or stun prey. They then turn and return to consume their "catch". Thresher sharks use their long tails to stun the shoaling fish. These sharks compact their prey school by swimming around them and splashing the water with their tails, often in pairs or small groups. They then strike them sharply with the upper lobe of their tails to stun them.[115] Spinner sharks charge vertically through the school, spinning on their axis with their mouths open and snapping all around. The shark's momentum at the end of these spiraling runs often carries it into the air.[116][117]
Some whales lunge feed on bait balls.[118] Lunge feeding is an extreme feeding method, where the whale accelerates from below the bait ball to a high velocity and then opens its mouth to a large gape angle. This generates the water pressure required to expand its mouth and engulf and filter a huge amount of water and fish. Lunge feeding by rorquals, a family of huge baleen whales that includes the blue whale, is said to be the largest biomechanical event on Earth.[119]
| More images | ||||
|---|---|---|---|---|
   
| ||||
Fisheries
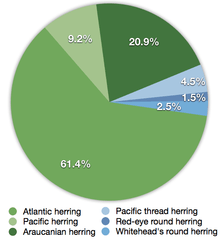
Green = Clupea herrings

Adult herring are harvested for their flesh and eggs, and they are often used as baitfish. The trade in herring is an important sector of many national economies. In Europe the fish has been called the "silver of the sea", and its trade has been so significant to many countries that it has been regarded as the most commercially important fishery in history.[120]
Environmental Defense have suggested that the Atlantic herring (Clupea harengus) fishery is an environmentally responsible fishery.[121]
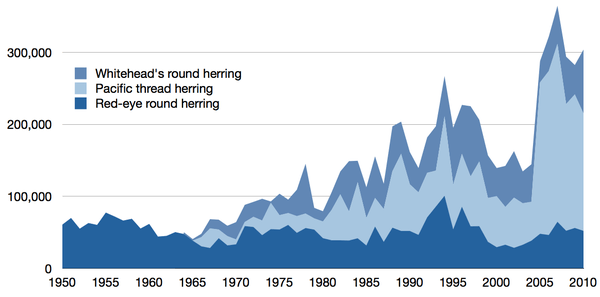
| Detailed time series |
|---|
As food
Herring has been a staple food source since at least 3000 B.C. There are numerous ways the fish is served and many regional recipes: eaten raw, fermented, pickled, or cured by other techniques, such as being smoked as kippers.
Herring are very high in the long-chain Omega-3 fatty acids EPA and DHA.[122] They are a source of vitamin D.
Water pollution influences the amount of herring that may be safely consumed. For example, large Baltic herring slightly exceeds recommended limits with respect to PCB and dioxin, although some sources point out that the cancer-reducing effect of omega-3 fatty acids is statistically stronger than the cancer-causing effect of PCBs and dioxins.[123] The contaminant levels depend on the age of the fish which can be inferred from their size. Baltic herrings larger than 17 cm may be eaten twice a month, while herrings smaller than 17 cm can be eaten freely.[124] Mercury in fish also influences the amount of fish that women who are pregnant or planning to be pregnant within the next one or two years may safely eat.
History
| Historical images |
|---|
Paintings     Herring boats     General history        |
See also
References
Notes
- 1 2 3 4 Based on data sourced from the relevant FAO Species Fact Sheets
- ↑ Cushing, David H (1975) Marine ecology and fisheries Cambridge University Press. ISBN 978-0-521-09911-0.
- ↑ Went, AEJ (1972) "The History of the International Council for the Exploration of the Sea". Proceedings of the Royal Society of Edinburgh. Section B. Biology, 73: 351–360.doi:10.1017/S0080455X0000240X
- 1 2 Pauly, Daniel (2004) Darwin's Fishes: An Encyclopedia of Ichthyology, Ecology, and Evolution Page 109, Cambridge University Press. ISBN 978-0-521-82777-5.
- ↑ "What's an oily fish?". Food Standards Agency. 2004-06-24.
- ↑ Herring Online Etymology Dictionary, Retrieved 10 April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Clupea bentincki" in FishBase. April 2012 version.
- ↑ Clupea bentincki (Norman, 1936) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Clupea bentincki". Integrated Taxonomic Information System. Retrieved April 2012.
- 1 2 Froese, Rainer and Pauly, Daniel, eds. (2012). "Clupea harengus" in FishBase. April 2012 version.
- ↑ Clupea harengus (Linnaeus, 1758) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Clupea harengus". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Herdson D and Priede I (2011). "Clupea harengus". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved 6 April 2012.
- ↑ Clupea pallasii (Valenciennes, 1847) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Clupea pallasii". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Clupeoides papuensis" in FishBase. April 2012 version.
- ↑ "Clupeoides papuensis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Allen G (2010). "Clupeoides papuensis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Dayella malabarica" in FishBase. April 2012 version.
- ↑ "Dayella malabarica". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Ali A and Raghavan R (2011). "Dayella malabarica". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Jenkinsia lamprotaenia" in FishBase. April 2012 version.
- ↑ "Jenkinsia lamprotaenia". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Jenkinsia lamprotaenia". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Gilchristella aestuaria" in FishBase. April 2012 version.
- ↑ "Gilchristella aestuaria". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Bills R (2007). "Gilchristella aestuaria". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Jenkinsia majua" in FishBase. April 2012 version.
- ↑ "Jenkinsia majua". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Munroe TA and Priede IG (2010). "Jenkinsia majua". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Etrumeus teres" in FishBase. April 2012 version.
- ↑ Etrumeus teres (Norman, 1936) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Etrumeus teres". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Spratellomorpha bianalis" in FishBase. April 2012 version.
- ↑ "Spratellomorpha bianalis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Loiselle, P; et al. (2011). "Spratellomorpha bianalis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Etrumeus whiteheadi" in FishBase. April 2012 version.
- ↑ Etrumeus whiteheadi (Wongratana, 1983) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Etrumeus whiteheadi". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Heemstra PC, Munroe TA and Priede IG (2011). "Etrumeus whiteheadi". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Jenkinsia parvula" in FishBase. April 2012 version.
- ↑ "Jenkinsia parvula". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Jenkinsia parvula". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthonema berlangai" in FishBase. April 2012 version.
- 1 2 "Opisthonema libertate". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T and Eschmeyer W (2010). "Opisthonema berlangai". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthonema medirastre" in FishBase. April 2012 version.
- ↑ "Opisthonema medirastre". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Opisthonema medirastre". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthonema libertate" in FishBase. April 2012 version.
- ↑ Opisthonema libertate (Günther, 1867) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Opisthonema libertate". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthonema bulleri" in FishBase. April 2012 version.
- ↑ "Opisthonema bulleri". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Opisthonema bulleri". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Lile nigrofasciata" in FishBase. April 2012 version.
- ↑ "Lile nigrofasciata". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T and Eschmeyer W (2010). "Lile nigrofasciata". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Denticeps clupeoides" in FishBase. April 2012 version.
- ↑ "Denticeps clupeoides". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Lalèyè P, Moelants T and Olaosebikan BD (2010). "Denticeps clupeoides". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Chirocentrodon bleekerianus" in FishBase. April 2012 version.
- ↑ "Chirocentrodon bleekerianus". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Priede IG (2010). "Chirocentrodon bleekerianus". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Lile gracilis" in FishBase. April 2012 version.
- ↑ "Lile gracilis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T, Eschmeyer W and Smith-Vaniz B (2010). "Lile gracilis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Harengula thrissina" in FishBase. April 2012 version.
- ↑ "Harengula thrissina". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T, Eschmeyer W and Smith-Vaniz B (2010). "Harengula thrissina". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Thrattidion noctivagus" in FishBase. April 2012 version.
- ↑ "Thrattidion noctivagus". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Moelants T (2010). "Thrattidion noctivagus". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Spratelloides gracilis" in FishBase. April 2012 version.
- ↑ "Spratelloides gracilis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Lile stolifera" in FishBase. April 2012 version.
- ↑ "Lile stolifera". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T and Eschmeyer W (2010). "Lile stolifera". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Sierrathrissa leonensis" in FishBase. April 2012 version.
- ↑ "Sierrathrissa leonensis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Moelants T and Olaosebikan BD (2010). "Sierrathrissa leonensis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthopterus macrops" in FishBase. April 2012 version.
- ↑ "Opisthopterus macrops". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Opisthopterus macrops". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthonema dovii" in FishBase. April 2012 version.
- ↑ "Opisthopterus dovii". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T, Eschmeyer W and Alvarado J (2010). "Opisthopterus dovii". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Ilisha fuerthii" in FishBase. April 2012 version.
- ↑ "Ilisha fuerthii". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T, Eschmeyer W and Alvarado J (2010). "Ilisha fuerthii". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Odontognathus panamensis" in FishBase. April 2012 version.
- ↑ "Odontognathus panamensis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A, Medina E and Bernal O (2010). "Odontognathus panamensis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Neoopisthopterus tropicus" in FishBase. April 2012 version.
- ↑ "Neoopisthopterus tropicus". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T and Eschmeyer W (2010). "Neoopisthopterus tropicus". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthopterus effulgens" in FishBase. April 2012 version.
- ↑ "Opisthopterus effulgens". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Iwamoto T, Eschmeyer W and Alvarado J (2010). "Opisthopterus effulgens". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Opisthopterus equatorialis" in FishBase. April 2012 version.
- ↑ "Opisthopterus equatorialis". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Cotto A (2010). "Opisthopterus equatorialis". IUCN Red List of Threatened Species. Version 2011.2. International Union for Conservation of Nature. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Chirocentrus dorab" in FishBase. April 2012 version.
- ↑ Chirocentrus dorab (Forsskål, 1775) FAO, Species Fact Sheet. Retrieved April 2012.
- ↑ "Chirocentrus dorab". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Chirocentrus nudus" in FishBase. April 2012 version.
- ↑ "Chirocentrus nudus". Integrated Taxonomic Information System. Retrieved April 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Coregonus artedi" in FishBase. April 2012 version.
- ↑ "Coregonus artedi". Integrated Taxonomic Information System. Retrieved April 2012.
- 1 2 Kils U (1992) The ATOLL Laboratory and other Instruments Developed at Kiel U.S. GLOBEC News, Technology Forum Number 8: 6-9.
- ↑ Biology of Copepods at Carl von Ossietzky University of Oldenburg
- 1 2 Friedrich W. Köster, et al. "Developing Baltic Cod Recruitment Models. I. Resolving Spatial And Temporal Dynamics Of Spawning Stock And Recruitment For Cod, Herring, And Sprat." Canadian Journal Of Fisheries & Aquatic Sciences 58.8 (2001): 1516. Academic Search Premier. Web. 21 Nov. 2011. p. 1516.
- ↑ Maris Plikshs, et al. "Developing Baltic Cod Recruitment Models. I. Resolving Spatial And Temporal Dynamics Of Spawning Stock And Recruitment For Cod, Herring, And Sprat." Canadian Journal Of Fisheries & Aquatic Sciences 58.8 (2001): 1516. Academic Search Premier. Web. 23 Nov. 2011, p.1517
- 1 2 3 Casini, Michele, Cardinale, Massimiliano, and Arrheni, Fredrik. "Feeding preferences of herring (Clupea harengus) and sprat (Sprattus sprattus) in the southern Baltic Sea." ICES Journal of Marine Science, 61 (2004): 1267-1277. Science Direct. Web. 22 November 2011. p. 1268.
- ↑ Seitz, J.C. Pelagic Thresher. Florida Museum of Natural History. Retrieved on December 22, 2008.
- ↑ Compagno, L.J.V. (1984). Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date. Rome: Food and Agricultural Organisation. pp. 466–468. ISBN 92-5-101384-5.
- ↑ "Carcharhinus brevipinna, Spinner Shark". MarineBio.org. Retrieved May 9, 2009.
- ↑ Reeves RR, Stewart BS, Clapham PJ and Powell J A (2002) National Audubon Society Guide to Marine Mammals of the World Chanticleer Press. ISBN 9780375411410.
- ↑ Potvin J and Goldbogen JA (2009) "Passive versus active engulfment: verdict from trajectory simulations of lunge-feeding fin whales Balaenoptera physalus J. R. Soc. Interface, 6(40): 1005–1025. doi:10.1098/rsif.2008.0492
- ↑ Herring, from Census of Marine Life, 2010.
- ↑ Eco-Best Fish - Safe for the environment, from Environmental Defense Fund, 2010.
- ↑ Cardiovascular Benefits Of Omega-3 Fatty Acids Reviewed
- ↑ Risks and benefits are clarified by food risk assessment - Finnish Food Safety Authority Evira
- ↑ Dietary advice on fish consumption - Finnish Food Safety Authority Evira
- ↑ River herring NEFSC, NOAA. Updated December 2006.
- ↑ Salisbury and Winchester Journal, 9 January 1792.
Bibliography
- Froese, Rainer, and Daniel Pauly, eds. (2006). Species of Clupea in FishBase. January 2006 version.
- Dewhurst HW (1834) Clupea harengis or the common herring In: The Natural History of the Order Cetacea, Oxford University. Pages 232–246.
- Geffen, Audrey J (2009) Advances in herring biology: from simple to complex, coping with plasticity and adaptability: ICES Journal of Marine Science, 66 (8): 1688–1695.
- Gilpen JB (1867) "On the common herring (Clupea elongata)" Proceedings and Transactions of the Nova-Scotian Institute of Natural Science, 1 (1): 4–11.
- O'Clair, Rita M. and O'Clair, Charles E., "Pacific herring," Southeast Alaska's Rocky Shores: Animals. pg. 343-346. Plant Press: Auke Bay, Alaska (1998). ISBN 0-9664245-0-6
- Stephenson RL (2001) The role of herring investigations in shaping fisheries science In F. Funk, J. Blackburn, D. Hay, A.J. Paul, R. Stephen- son, R. Toresen, and D. Witherell (eds.) Herrings: Expectations for a New Millennium, Alaska Sea Grant College Program. AK-SG-01-04. Pages 1–20. ISBN 1-56612-070-5.
- Stephenson, R. L., Melvin, G. D., and Power, M. J. (2009) "Population integrity and connectivity in Northwest Atlantic herring: a review of assumptions and evidence" ICES Journal of Marine Science, 66: 1733–1739.
- Whitehead PJP, Nelson GJ and Wongratana T (1988) FAO species catalogue, volume 2: Clupeoid Fishes of the World, Suborder Clupeoidei FAO Fisheries Synopsis 125, Rome. ISBN 92-5-102340-9. Download ZIP (16 MB)
Further reading
- Baltic Fisheries Cooperation Committee (1995) Utilization and Marketing of Baltic Herring Nordic Council of Ministers. ISBN 9789291207749.
- Bigelow HB and Schroeder WC (1953) Fishes of the Gulf of Maine Pages 88–100, Fishery Bulletin 74(53), NOAA. pdf version
- Dodd JS (1752) An essay toward a natural history of the herring Original from the New York Public Library.
- Mitchell JM (1864) The herring: its natural history and national importance Edmonston and Douglas. Original from the University of Wisconsin.
- Postan MM, Miller E and Habakkuk HJ (1987) The Cambridge Economic History of Europe: Trade and industry in the Middle Ages Cambridge University Press. ISBN 9780521087094.
- Poulsen B (2008) Dutch Herring: An Environmental History, C. 1600-1860 Amsterdam University Press. ISBN 9789052603049.
- Samuel AM (1918) The herring: its effect on the history of Britain J. Murray. Original from the University of Michigan.
- Stephenson F (2007) Herring Fishermen: Images of an Eastern North Carolina Tradition The History Press. ISBN 9781596292697.
- Waters B (1809) Letters upon the subject of the herring fishery: addressed to the secretary of the Honourable the Board for the Herring Fishery at Edinburgh, to which is added, a petition to the lords of the treasury on the same subject Original from Harvard University.
External links
| Wikibooks Cookbook has a recipe/module on |
| Wikimedia Commons has media related to Clupea harengus. |
- Herring "communicate" by flatulence from national geographic
- Atlantic Herring from the Gulf of Maine Research Institute
- Nutrition Facts for Herring
- Prospecting herring waste - from ScienceNordic
- PNAS Population-scale sequencing reveals genetic differentiation due to local adaptation in Atlantic herring.
| ||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||





.png)
