Osteogenesis imperfecta
| Osteogenesis imperfecta | |
|---|---|
 The classic blue sclerae of a person with osteogenesis imperfecta | |
| Classification and external resources | |
| Specialty | Pediatrics, medical genetics, osteology |
| ICD-10 | Q78.0 |
| ICD-9-CM | 756.51 |
| OMIM | 166200 |
| DiseasesDB | 9342 |
| MedlinePlus | 001573 |
| eMedicine | ped/1674 |
| Patient UK | Osteogenesis imperfecta |
| MeSH | D010013 |
| Orphanet | 666 |
Osteogenesis imperfecta (OI), also known as brittle bone disease or Lobstein syndrome,[1] is a congenital bone disorder characterized by brittle bones that are prone to fracture. OI may also present with shorter height, neurological features including communicating hydrocephalus, basilar invagination, and seizures,[2] blue sclerae, hearing loss, or other complications. The fractures themselves can cause acute or chronic pain, reduced quality of life, and depression.[3]
People with OI are born with defective connective tissue, or without the ability to make it, usually because of a deficiency of type I collagen.[4] Eight types of OI can be distinguished. Most cases are caused by mutations in the COL1A1 and COL1A2 genes, both of which code for type I collagen. Diagnosis of OI is based on the clinical features and may be confirmed by collagen or DNA testing.
There is no cure for OI. Treatment is aimed at increasing overall bone strength to prevent fracture and maintain mobility. Treatment includes bisphosphonates, surgery, physical therapy, and physical aids.
OI occurs in about one per 20,000 live births. The frequency of OI doesn't change across groups, but certain types are more common in certain groups.
Classification
Of the eight different types of OI, type I is the most common, though the symptoms vary from person to person.
| Type | Description | Gene | OMIM | Mode of inheritance |
| I | mild | Null COL1A1 allele | 166240 (IA), 166200 (IB) | autosomal dominant, 60% de novo[5] |
| II | severe and usually lethal in the perinatal period | COL1A1, COL1A2, | 166210 (IIA), 610854 (IIB) | autosomal dominant, ~100% de novo[5] |
| III | considered progressive and deforming | COL1A1, COL1A2 | 259420 | autosomal dominant, ~100% de novo[5] |
| IV | deforming, but with normal sclerae most of the time | COL1A1, COL1A2 | 166220 | autosomal dominant, 60% de novo[5] |
| V | shares the same clinical features of IV, but has unique histologic findings ("mesh-like") | IFITM5 | 610967 | autosomal dominant[5][6] |
| VI | shares the same clinical features of IV, but has unique histologic findings ("fish scale") | SERPINF1 | 610968 | autosomal recessive[5] |
| VII | associated with cartilage associated protein | CRTAP | 610682 | autosomal recessive[5] |
| VIII | severe to lethal, associated with the protein leprecan | LEPRE1 | 610915 | autosomal recessive |
Type I

Collagen is of normal quality but is produced in insufficient quantities:
- Bones fracture easily
- Slight spinal curvature
- Loose joints
- Poor muscle tone
- Discoloration of the sclera (whites of the eyes), usually giving them a blue-gray color. The blue-gray color of the sclera is due to the underlying choroidal veins which show through. This is due to the sclera being thinner than normal because the defective Type I collagen is not forming correctly.
- Early loss of hearing in some children[7]
- Slight protrusion of the eyes
IA and IB are defined to be distinguished by the absence/presence of dentinogenesis imperfecta (characterized by opalescent teeth; absent in IA, present in IB). Life expectancy is slightly reduced compared to the general population due to the possibility of fatal bone fractures and complications related to OI Type I such as basilar invagination.
Type II
Collagen is not of a sufficient quality or quantity
- Most cases die within the first year of life due to respiratory failure or intracerebral hemorrhage
- Severe respiratory problems due to underdeveloped lungs
- Severe bone deformity and small stature
Type II can be further subclassified into groups A, B, and C, which are distinguished by radiographic evaluation of the long bones and ribs. Type IIA demonstrates broad and short long bones with broad and beaded ribs. Type IIB demonstrates broad and short long bones with thin ribs that have little or no beading. Type IIC demonstrates thin and longer long bones with thin and beaded ribs.
Type III
Collagen improperly formed, enough collagen is made but it is defective
- Bones fracture easily, sometimes even before birth
- Bone deformity, often severe
- Respiratory problems possible
- Short stature, spinal curvature and sometimes barrel-shaped rib cage
- Triangular face[8]
- Loose joints (double jointed)
- Poor muscle tone in arms and legs
- Discolouration of the sclera (the 'whites' of the eyes are blue)
- Early loss of hearing possible
Type III is distinguished among the other classifications as being the "progressive deforming" type, wherein a neonate presents with mild symptoms at birth and develops the aforementioned symptoms throughout life. Lifespans may be normal, albeit with severe physical handicapping.
Type IV
Collagen quantity is sufficient but is not of a high enough quality
- Bones fracture easily, especially before puberty
- Short stature, spinal curvature, and barrel-shaped rib cage
- Bone deformity is mild to moderate
- Early loss of hearing
Similar to Type I, Type IV can be further subclassified into types IVA and IVB characterized by absence (IVA) or presence (IVB) of dentinogenesis imperfecta.
Type V
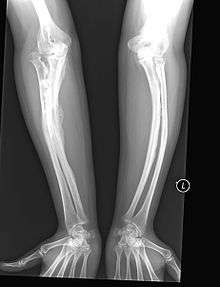

Having the same clinical features as Type IV, it is distinguished histologically by "mesh-like" bone appearance. Further characterized by the "V triad" consisting of a) radio-opaque band adjacent to growth plates, b) hypertrophic calluses at fracture sites, and c) calcification of the radio-ulnar interosseous membrane.[9]
OI Type V leads to calcification of the membrane between the two forearm bones, making it difficult to turn the wrist. Another symptom is abnormally large amounts of repair tissue (hyperplasic callus) at the site of fractures. Other features of this condition include radial head dislocation, long bone bowing, and mixed hearing loss.
At least some cases of this type are caused by mutations in the IFITM5 gene.[6]
Type VI
With the same clinical features as Type IV, it is distinguished histologically by "fish-scale" bone appearance. Type VI has recently been found to be caused by a loss of function mutation in the SERPINF1 gene. SERPINF1, a member of the serpin family, is also known as pigment epithelium derived factor (PEDF), the most powerful endogenous antiangiogenic factor in mammals.
Type VII
In 2006, a recessive form called "Type VII" was discovered (phenotype severe to lethal). Thus far it seems to be limited to a First Nations people in Quebec.[10] Mutations in the gene CRTAP causes this type.[11]
Type VIII
OI caused by mutation in the gene LEPRE1 is classified as type VIII.[11]
Other genes
A family with recessive osteogenesis imperfecta has been reported to have a mutation in the TMEM38B gene on chromosome 9.[12] This gene encodes TRIC-B, a component of TRIC, a monovalent cation-specific channel involved in calcium release from intracellular stores.
It is extremely likely that there are other genes associated with this disease that have yet to be reported.
Pathophysiology
People with OI are born with defective connective tissue, or without the ability to make it, usually because of a deficiency of Type-I collagen.[4] This deficiency arises from an amino acid substitution of glycine to bulkier amino acids in the collagen triple helix structure. The larger amino acid side-chains create steric hindrance that creates a bulge in the collagen complex, which in turn influences both the molecular nanomechanics and the interaction between molecules, which are both compromised.[13] As a result, the body may respond by hydrolyzing the improper collagen structure. If the body does not destroy the improper collagen, the relationship between the collagen fibrils and hydroxyapatite crystals to form bone is altered, causing brittleness.[14] Another suggested disease mechanism is that the stress state within collagen fibrils is altered at the locations of mutations, where locally larger shear forces lead to rapid failure of fibrils even at moderate loads as the homogeneous stress state found in healthy collagen fibrils is lost.[13] These recent works suggest that OI must be understood as a multi-scale phenomenon, which involves mechanisms at the genetic, nano-, micro- and macro-level of tissues.
As a genetic disorder, OI has historically been viewed as an autosomal dominant disorder of type I collagen. Most cases have been caused by mutations in the COL1A1 and COL1A2 genes. In the past several years, there has been the identification of autosomal recessive forms.[15] Most people with OI receive it from a parent but in 35% of cases it is an individual (de novo or "sporadic") mutation.
Diagnosis
There is no definitive test for OI. The diagnosis is usually suggested by the occurrence of bone fractures with little trauma and the presence of other clinical features. A skin biopsy can be performed to determine the structure and quantity of type I collagen. DNA testing can confirm the diagnosis, however, it cannot exclude it because not all mutations causing OI are known and/or tested for. OI type II is often diagnosed by ultrasound during pregnancy, where already multiple fractures and other characteristic features may be present. Relative to control, OI cortical bone shows increased porosity, canal diameter, and connectivity in micro-computed tomography.[16]
An important differential diagnosis of OI is child abuse, as both may present with multiple fractures in various stages of healing. Differentiating them can be difficult, especially when no other characteristic features of OI are present. Other differential diagnoses include rickets, osteomalacia, and other rare skeletal syndromes.
Treatment
There is no cure for OI. Treatment is aimed at increasing overall bone strength to prevent fracture and maintain mobility. Bisphosphonates can increase bone mass, and reduce bone pain and fracture. In severe cases, bones are surgically corrected, and rods are placed inside the bones, particularly to enable infants to learn to walk.
Bone infections are treated as and when they occur with the appropriate antibiotics and antiseptics.
Bisphosphonates
In 1998, a clinical trial demonstrated the effectiveness of intravenous pamidronate, a bisphosphonate which had previously been used in adults to treat osteoporosis. In severe OI, pamidronate reduced bone pain, prevented new vertebral fractures, reshaped previously fractured vertebral bodies, and reduced the number of long-bone fractures.[17]
Although oral bisphosphonates are more convenient and cheaper, they are not absorbed as well, and intravenous bisphosphonates are generally more effective, although this is under study. Some studies have found oral and intravenous bisphosphonates, such as oral alendronate and intravenous pamidronate, equivalent.[18] In a trial of children with mild OI, oral risedronate increased bone mineral densities, and reduced nonvertebral fractures. However, it did not decrease new vertebral fractures.[19][20]
Bisphosphonates are less effective for OI in adults.[21]
Surgery
Metal rods can be surgically inserted in the long bones to improve strength, a procedure developed by Harold A. Sofield, MD, at Shriners Hospitals for Children in Chicago. During the late 1940s, Sofield, Chief of Staff at Shriners Hospitals in Chicago, worked there with large numbers of children with OI and experimented with various methods to strengthen the bones in these children.[22] In 1959, with Edward A. Miller, MD, Sofield wrote a seminal article describing a solution that seemed radical at the time: the placement of stainless steel rods into the intramedullary canals of the long bones to stabilize and strengthen them. His treatment proved extremely useful in the rehabilitation and prevention of fractures; it was adopted throughout the world and still forms the basis for orthopedic treatment of OI.
Spinal fusion can be performed to correct scoliosis, although the inherent bone fragility makes this operation more complex in OI patients. Surgery for basilar impressions can be carried out if pressure being exerted on the spinal cord and brain stem is causing neurological problems.
Physiotherapy
Physiotherapy is used to strengthen muscles and improve motility in a gentle manner, while minimizing the risk of fracture. This often involves hydrotherapy and the use of support cushions to improve posture. Individuals are encouraged to change positions regularly throughout the day to balance the muscles being used and the bones under pressure.
Children often develop a fear of trying new ways of moving due to movement being associated with pain. This can make physiotherapy difficult to administer to young children.
Physical aids
With adaptive equipment such as crutches, wheelchairs, splints, grabbing arms, or modifications to the home, many individuals with OI can obtain a significant degree of autonomy.
History
The condition, or types of it, has had various other names over the years and in different nations. Among some of the most common alternatives are Ekman-Lobstein syndrome, Vrolik syndrome, and the colloquial glass-bone disease. The name osteogenesis imperfecta dates to at least 1895[23] and has been the usual medical term in the 20th century to present. The current four type system began with Sillence in 1979.[24] An older system deemed less severe types "osteogenesis imperfecta tarda" while more severe forms were deemed "osteogenesis imperfecta congenita."[25] As this did not differentiate well, and all forms are congenital, this has since fallen out of favour.
The condition has been found in an ancient Egyptian mummy from 1000 BC. The Norse king Ivar the Boneless may have had this condition, as well. The earliest studies of it began in 1788 with the Swede Olof Jakob Ekman. He described the condition in his doctoral thesis and mentioned cases of it going back to 1678. In 1831, Edmund Axmann described it in himself and two brothers. Jean Lobstein dealt with it in adults in 1833. Willem Vrolik did work on the condition in the 1850s. The idea that the adult and newborn forms were the same came in 1897 with Martin Benno Schmidt.[26]
Epidemiology
In the United States, the incidence of osteogenesis imperfecta is estimated to be one per 20,000 live births.[27] An estimated 20,000 to 50,000 people are affected by OI in the United States.
Frequency is approximately the same across groups, but for unknown reasons, the Shona and Ndebele of Zimbabwe seem to have a higher proportion of Type III to Type I than other groups.[28] However, a similar pattern was found in segments of the Nigerian and South African populations. In these varied cases, the total number of OIs of all four types was roughly the same as any other ethnicity.
Society and culture
The Brittle Bone Society is a UK charity that supports people with the condition.
Figures in film, television, video games and novels depicted as having osteogenesis imperfecta include:
- (2013) Robbie Novak a.k.a. Kid President, a motivational speaker and YouTube personality, has the disorder.[29] He often comments on his own ability to overcome it.[30]
- In M. Night Shyamalan's 2000 film Unbreakable, Samuel L. Jackson's character Elijah Price was born with type I Osteogenesis imperfecta.[31]
- Jeff "Joker" Moreau, a fictional character from the Mass Effect video game series voiced by Seth Green. Given that the series is set in the fictional future, he is able to take medications to help with his disease and is a pilot. The medication, along with a mix of Cerberus implants and braces, allow him to walk and dance, though not as fluidly as normal humans. [32]
Other animals
In dogs, OI is an autosomal-recessive condition. In Golden Retrievers, it is caused by a mutation in the COL1A1, and in Beagles, the COL1A2. A separate mutation in the SERPINH1 gene has been found to cause the condition in Dachshunds.[33] Several mouse models of OI have been described, whereby the abnormal gait 2 (AGA2) mouse line exhibits severe skeletal[34] and cardio-pulmonary[35] phenotypes due to a carboxy-terminus mutation in the COL1A1 gene in the mouse.
References
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. Page 517. ISBN 0-7216-2921-0.
- ↑ Charnas, L. R.; Marini, J. C. (1993-12-01). "Communicating hydrocephalus, basilar invagination, and other neurologic features in osteogenesis imperfecta". Neurology 43 (12): 2603–2608. ISSN 0028-3878. PMID 8255464.
- ↑ "Pain Management - Osteogenesis Imperfecta Foundation | OIF.org". www.oif.org. Retrieved 2016-01-06.
- 1 2 Rauch F, Glorieux FH (2004). "Osteogenesis imperfecta". Lancet 363 (9418): 1377–85. doi:10.1016/S0140-6736(04)16051-0. PMID 15110498.
- 1 2 3 4 5 6 7 Steiner RD, Pepin MG, Byers PH, Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP (January 28, 2005). "Osteogenesis Imperfecta". PMID 20301472. Retrieved 26 March 2012.
- 1 2 Shapiro JR, Lietman C, Grover M, Lu JT, Nagamani SC, Dawson BC, Baldridge DM, Bainbridge MN, Cohn DH, Blazo M, Roberts TT, Brennen FS, Wu Y, Gibbs RA, Melvin P, Campeau PM, Lee BH (2013). "Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation". J. Bone Miner. Res. 28 (7): 1523–30. doi:10.1002/jbmr.1891. PMC 3688672. PMID 23408678.
- ↑ Fuller E, Lin V, Bell M, Bharatha A, Yeung R, Aviv RI, Symons SP (2011). "Case of the month #171: osteogenesis imperfecta of the temporal bone". Can Assoc Radiol J 62 (4): 296–8. doi:10.1016/j.carj.2010.04.002. PMID 22018338.
- ↑ Page 771 in: Chen, Harold (2006). Atlas of genetic diagnosis and counseling. Totowa, NJ: Humana Press. ISBN 1-58829-681-4.
- ↑ Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, Lalic L, Glorieux DF, Fassier F, Bishop NJ (2000). "Type V osteogenesis imperfecta: a new form of brittle bone disease". J. Bone Miner. Res. 15 (9): 1650–8. doi:10.1359/jbmr.2000.15.9.1650. PMID 10976985.
- ↑ "Recessive Form of OI Discovered by Foundation-funded Researcher" (PDF).
- 1 2 Genetics Home Reference: Genetic Conditions > Osteogenesis imperfecta (Reviewed November 2007)
- ↑ Volodarsky M, Markus B, Cohen I, Staretz-Chacham O, Flusser H, Landau D, Shelef I, Langer Y, Birk OS (2013) A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum Mutat doi:10.1002/humu.22274
- 1 2 Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ (2009). "Molecular and mesoscale disease mechanisms of Osteogenesis Imperfecta". Biophysical Journal 97 (3): 857–865. doi:10.1016/j.bpj.2009.04.059. PMC 2718154. PMID 19651044.
- ↑ "Osteogenesis Imperfecta Foundation: Fast Facts". Retrieved 2007-07-05.
- ↑ Drögemüller C, Becker D, Brunner A, Haase B, Kircher P, Seeliger F, Fehr M, Baumann U, Lindblad-Toh K, Leeb T (2009). Barsh, Gregory S, ed. "A Missense Mutation in the SERPINH1 Gene in Dachshunds with Osteogenesis Imperfecta". PLoS Genetics 5 (7): e1000579. doi:10.1371/journal.pgen.1000579. PMC 2708911. PMID 19629171.
- ↑ http://proceedings.spiedigitallibrary.org/proceeding.aspx?articleid=1674625
- ↑ Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R (1998). "Cyclic administration of pamidronate in children with severe osteogenesis imperfecta". N. Engl. J. Med. 339 (14): 947–52. doi:10.1056/NEJM199810013391402. PMID 9753709.Free full text
- ↑ DiMeglio LA, Peacock M (2006). "Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta". J. Bone Miner. Res. 21 (1): 132–40. doi:10.1359/JBMR.051006. PMID 16355282.
- ↑ Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial, Lancet, 6 August 2013
- ↑ Oral bisphosphonates for paediatric osteogenesis imperfecta? Lancet August 6, 2013
- ↑ Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina-Granade G, Duboeuf F, Garnero P, Arlot M, Raynal C, Meunier PJ (2006). "Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial". J. Bone Miner. Res. 21 (2): 300–6. doi:10.1359/JBMR.051015. PMID 16418786.
- ↑ "Chicago Shriners Hospital - Osteogenesis imperfecta". Retrieved 2007-07-05.
- ↑ K. Buday, Beiträge zur Lehre von der Osteogenesis imperfecta (1895)
- ↑ Sillence DO, Senn A, Danks DM (1979). "Genetic heterogeneity in osteogenesis imperfecta". J. Med. Genet. 16 (2): 101–16. doi:10.1136/jmg.16.2.101. PMC 1012733. PMID 458828.
- ↑ "Osteogenesis Imperfecta Foundation: Glossary". Retrieved 2007-07-05.
- ↑ synd/1743 at Who Named It?
- ↑ eMedicine > Osteogenesis Imperfecta Author: Horacio Plotkin. Updated: March 2, 2010
- ↑ Viljoen D, Beighton P (1987). "Osteogenesis imperfecta type III: an ancient mutation in Africa?". Am. J. Med. Genet. 27 (4): 907–12. doi:10.1002/ajmg.1320270417. PMID 3425600.
- ↑ "The inspiring life of the "Kid President"". CBS News. Retrieved September 27, 2013.
- ↑ Soul Pancake. "The True Story of Kid President." YouTube. Posted 2013-02-07. Retrieved 2013-09-27.
- ↑ http://www.imdb.com/title/tt0217869/
- ↑ http://masseffect.neoseeker.com/wiki/Jeff_%22Joker%22_Moreau
- ↑ Eckardt J, Kluth S, Dierks C, Philipp U, Distl O (2013). "Population screening for the mutation associated with osteogenesis imperfecta in dachshunds". Vet. Rec. 172 (14): 364. doi:10.1136/vr.101122. PMID 23315765.
- ↑ Lisse TS, Thiele F, Fuchs H, Hans W, Przemeck GK, Abe K, Rathkolb B, Quintanilla-Martinez L, Hoelzlwimmer G, Helfrich M, Wolf E, Ralston SH, Hrabé de Angelis M. (Feb 2008). "ER stress-mediated apoptosis in a new mouse model of osteogenesis imperfecta.". PLoS Genet. 4 (2): e7. doi:10.1371/journal.pgen.0040007. PMC 2222924. PMID 18248096.
- ↑ Thiele F, Cohrs CM, Flor A, Lisse TS, Przemeck GK, Horsch M, Schrewe A, Gailus-Durner V, Ivandic B, Katus HA, Wurst W, Reisenberg C, Chaney H, Fuchs H, Hans W, Beckers J, Marini JC, Hrabé de Angelis M. (Aug 2012). "Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms.". Hum Mol Genet. 21 (16): 3535–45. doi:10.1093/hmg/dds183. PMC 3406754. PMID 22589248.
External links
| Wikimedia Commons has media related to Osteogenesis imperfecta. |
- GeneReview/NCBI/NIH/UW entry on Osteogenesis Imperfecta
- synd/1743 at Who Named It?
- Osteogenesis Imperfecta Overview NIH Osteoporosis and Related Bone Diseases ~ National Resource Center
- Type V Research, Osteogenesis Imperfecta association
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||