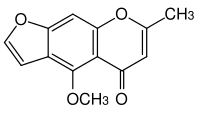
Visnagin
 | |
| Names | |
|---|---|
| IUPAC name
4-Methoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one[1] | |
| Other names
Visnacorin; Khella; Desmethoxykhellin; 5-Methoxy-2-methylfuranochromone; 5-Methoxy-2-methyl-6,7-furanochrome | |
| Identifiers | |
| 82-57-5 | |
| 5-19-06-00030 | |
| ChemSpider | 6460 |
| EC Number | 201-430-3 |
| 234955 | |
| Jmol interactive 3D | Image |
| PubChem | 24855274 |
| RTECS number | LV1420000 |
| |
| Properties | |
| C13H10O4 | |
| Molar mass | 230.22 g·mol−1 |
| Appearance | Solid |
| Melting point | 144 to 145 °C (291 to 293 °F; 417 to 418 K) |
| soluble | |
| Solubility | ethanol |
| Hazards | |
| Main hazards | Harmful by ingestion |
| Safety data sheet | |
| GHS pictograms |  |
| GHS signal word | WARNING |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
832 mg/kg (oral, rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Visnagin is an organic chemical compound with the molecular formula C13H10O4 It is a furanochromone, a compound derivative of chromone (1,4-benzopyrone) and furan.
History
Ammi visnaga, the main source for visnagin, has been used in traditional medicine in the Middle East to ease urinary tract pain associated with kidney stones and to promote stone passage.[2]
Occurrences
Visnagin naturally occurs in Ammi visnaga, a species of flowering plant in the carrot family known by many common names, including bisnaga, toothpickweed, and khella. Visnagin-containing khella seeds are usually found mainly in Middle East countries such as Egypt and Turkey and also in Northern African countries such as Morocco. Visnagin can be extracted directly from khella seeds.
Synthesis
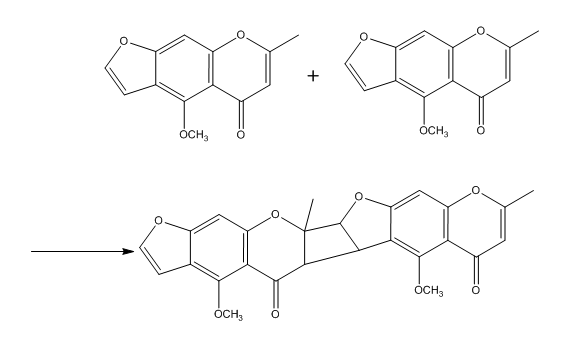
Modified synthesis of the naturally occurring visnagin is reported. Starting from phloroghrcin aldehyde, and building on the 2-methyl-y-pyrone, 2-methyl-5,7-dihydroxy-dfo-yl-chromone was obtained. Construction of the furan moiety was realized by a conventional method through the 7-carboxymethoxy ether giving S-norvisnagin which can be methylated to visnagin.[3]
Reactions
Condensation
Visnagin analogs can be synthesized through the condensation of visnagone with esters and sodium. This leads to the product of 2-ethyl, 2-(3'-pyridyl) visnagin analog (50c).[4]
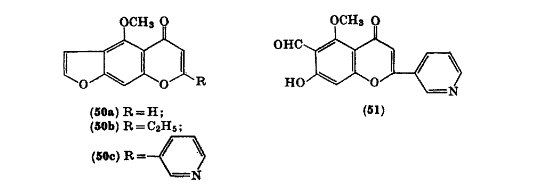 [4]
[4]
Oligomerization
Visnagin molecules can go over an oligomerization to form a chain of visnagin molecules.[5]
Animal study
Visnagin has biological activity in animal models as a vasodilator and reduces blood pressure by inhibiting calcium influx into the cell.[6] In rats, visnagin prevents the formation of kidney stones by prolonging the induction time of nucleation of crystals.[7][8]
On December 8th, 2014 it was reported that "visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase."[9]
References
- ↑ Visnacorin, ChemSpider
- ↑ Haug, Karin G.; Weber, Benjamin; Hochhaus, Guenther; Butterweck, Veronika (2012). "Nonlinear pharmacokinetics of visnagin in rats after intravenous bolus administration". European Journal of Pharmaceutical Sciences 45 (1–2): 79–89. doi:10.1016/j.ejps.2011.10.023. PMID 22085634.
- ↑ Badawi, M.M.; Fayez, M.B.E. (1965). "Natural chromones—I". Tetrahedron 21 (10): 2925. doi:10.1016/S0040-4020(01)98378-4.
- 1 2 Mustafa, Ahmed (2009-09-15). "The Chemistry of Heterocyclic Compounds, Furopyrans and Furopyrones". ISBN 9780470188354.
- ↑ Pradhan, Padmanava; Banerji, Asoke (1998). "Novel cyclobutane fused furochromone oligomers from the seeds of Pimpinella monoica Dalz". Tetrahedron 54 (48): 14541. doi:10.1016/S0040-4020(98)00913-2.
- ↑ Lee, Jin-Koo; Jung, Jun-Sub; Park, Sang-Hee; Park, Soo-Hyun; Sim, Yun-Beom; Kim, Seon-Mi; Ha, Tal-Soo; Suh, Hong-Won (2010). "Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells". Archives of Pharmacal Research 33 (11): 1843–50. doi:10.1007/s12272-010-1117-1. PMID 21116788.
- ↑ http://ull.chemistry.uakron.edu/erd/Chemicals/14000/12765.html
- ↑ Abdel-Aal, E.A.; Daosukho, S.; El-Shall, H. (2009). "Effect of supersaturation ratio and Khella extract on nucleation and morphology of kidney stones". Journal of Crystal Growth 311 (9): 2673. doi:10.1016/j.jcrysgro.2009.02.027.
- ↑ Liu, Y.; Asnani, A.; Zou, L.; Bentley, V. L.; Yu, M.; Wang, Y.; Dellaire, G.; Sarkar, K. S.; Dai, M.; Chen, H. H.; Sosnovik, D. E.; Shin, J. T.; Haber, D. A.; Berman, J. N.; Chao, W.; Peterson, R. T. (10 December 2014). "Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase". Science Translational Medicine 6 (266): 266ra170–266ra170. doi:10.1126/scitranslmed.3010189.