Vinyl
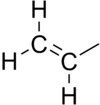
In chemistry, vinyl or ethenyl[1] is the functional group −CH=CH2, namely the ethylene molecule (H2C=CH2) minus one hydrogen atom. The name is also used for any compound containing that group, namely R−CH=CH2 where R is any other group of atoms.
An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as vinyl.

Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called divinyl benzene.)
Etymology
The etymology of vinyl is the Latin vinum = "wine", because of its relationship with alcohol (in its original sense of ethyl alcohol). The term "vinyl" was coined by the German chemist Hermann Kolbe in 1851.[2]
Vinyl polymers
Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. In these polymers, the double bonds of the vinyl monomers turn into single bonds and the different monomers are joined by single bonds. Vinyl groups do not exist in vinyl polymer; the term refers to the precursor. It is sometimes important to ascertain the absence of unreacted vinyl monomer in the final product when the monomer is toxic or reduces the performance of the plastic. The following table gives some examples of vinyl polymers.
| Monomer example | Example of resulting polymer |
|---|---|
| Vinyl chloride | Polyvinyl chloride (PVC) |
| Vinyl fluoride | Polyvinyl fluoride (PVF) |
| Vinyl acetate | Polyvinyl acetate (PVAc) |
The vinylidene and vinylene derivatives can polymerize in the same manner.
Reactivity
Vinyl derivatives are alkenes. If activated by an adjacent group, the increased polarization of the bond gives rise to characteristic reactivity, which is termed vinylogous:
- In allyl compounds, where the next carbon is saturated but substituted once, allylic rearrangement and related reactions are observed.
- Allyl Grignard reagents can attack with the vinyl end first.
- If next to an electron-withdrawing group, conjugate addition (Michael addition) occurs.
Vinyl organometallics, e.g. vinyl lithium, participate in coupling reactions such as in Negishi coupling.
Vinyl gloves in medicine
When used as medical gloves, due to vinyl gloves having less flexibility and elasticity, several guidelines recommend either latex or nitrile gloves for clinical care and procedures that require manual dexterity and/or that involve patient contact for more than a brief period.[3]
Vinyl gloves show poor resistance to many chemicals, including glutaraldehyde based products and alcohols used in formulation of disinfectants for swabbing down work surfaces or in hand rubs.[3]
Allergies
Several publications have highlighted cases of skin reactions due to chemical additives used in the manufacturing process of vinyl gloves:
- Bisphenol A, which is used as an antioxidant in PVC plastics and as an inhibitor of end polymerization in PVC, has been identified as a cause of some cases of allergic contact dermatitis.
- Exacerbation of hand dermatitis while using PVC gloves was noted in 8 patients who were allergic to benzisothiazolinone, a biocide widely used in the manufacture of disposable PVC gloves.
- Other studies identified additional chemical agents, such as an adipic polyester26, propylene glycol compound
and ethylhexylmaleate27, as a cause of allergic contact dermatitis in vinyl gloves.[3]
See also
References
- ↑ IUPAC Provisional Recommendations 2004 Chapter 5
- ↑ H. Kolbe (1851), "On the chemical constitution and nature of organic radicals," The Quarterly Journal of the Chemical Society of London, 3 (4) : 369-405; see footnote on page 376.
- 1 2 3 "Vinyl Gloves: Causes For Concern" (PDF). Ansell (glove manufacturer). Retrieved 17 November 2015.