Ventricular fibrillation
| Ventricular fibrillation | |
|---|---|
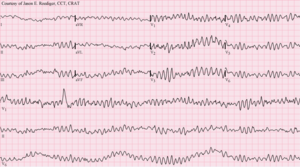 12-lead ECG of ventricular fibrillation | |
| Classification and external resources | |
| Specialty | Cardiology |
| ICD-10 | I49.0 |
| ICD-9-CM | 427.41 |
| DiseasesDB | 13798 |
| MedlinePlus | 007200 |
| Patient UK | Ventricular fibrillation |
| MeSH | D014693 |
Ventricular fibrillation (V-fib or VF) is a condition in which there is uncoordinated contraction of the cardiac muscle of the ventricles in the heart, making them quiver rather than contract properly. Ventricular fibrillation is the most commonly identified arrhythmia in cardiac arrest patients.[1] While there is some activity, the lay person is usually unable to detect it by palpating (feeling) the major pulse points of the carotid and femoral arteries. Such an arrhythmia is only confirmed by electrocardiography. Ventricular fibrillation is a medical emergency that requires prompt Advanced Life Support interventions. If this arrhythmia continues for more than a few seconds, it will likely degenerate further into asystole ("flatline"). This condition results in cardiogenic shock and cessation of effective blood circulation. As a consequence, sudden cardiac death (SCD) will result in a matter of minutes. If the patient is not revived after a sufficient period (within roughly 5 minutes at room temperature), the patient could sustain irreversible brain damage and possibly become brain-dead, due to the effects of cerebral hypoxia. On the other hand, death often occurs if sinus rhythm is not restored within 90 seconds of the onset of VF, especially if it has degenerated further into asystole.
Signs and symptoms
Ventricular fibrillation is a cause of cardiac arrest and sudden cardiac death. The ventricular muscle twitches randomly rather than contracting in a coordinated fashion (from the apex of the heart to the outflow of the ventricles), and so the ventricles fail to pump blood into the arteries and systemic circulation. Ventricular fibrillation is a sudden lethal arrhythmia responsible for many deaths in the Western world, and it is mostly caused by ischemic heart disease. While most episodes occur in diseased hearts, others can afflict normal hearts as well.
The symptoms can include chest pain, tachycardia, dizziness, nausea, and shortness of breath.[2]
Despite considerable research, the underlying nature of ventricular fibrillation is still not completely understood.
Cause

AC-1: imperceptible
AC-2: perceptible but no muscle reaction
AC-3: muscle contraction with reversible effects
AC-4: possible irreversible effects
AC-4.1: up to 5% probability of ventricular fibrillation
AC-4.2: 5-50% probability of fibrillation
AC-4.3: over 50% probability of fibrillation
Abnormal automaticity
Automaticity is a measure of the propensity of a fiber to initiate an impulse spontaneously. The product of a hypoxic myocardium can be hyperirritable myocardial cells. These may then act as pacemakers. The ventricles are then being stimulated by more than one pacemaker. Scar and dying tissue is inexcitable, but around these areas usually lies a penumbra of hypoxic tissue that is excitable. Ventricular excitability may generate re-entry ventricular arrhythmia.
It is interesting to note that most cardiac myocardial cells with an associated increased propensity to arrhythmia development have an associated loss of membrane potential. That is, the maximum diastolic potential is less negative and therefore exists closer to the threshold potential. Cellular depolarisation can be due to a raised external concentration of potassium ions K+, a decreased intracellular concentration of sodium ions Na+, increased permeability to Na+, or a decreased permeability to K+. The ionic basic automaticity is the net gain of an intracellular positive charge during diastole in the presence of a voltage-dependent channel activated by potentials negative to –50 to –60 mV.
Myocardial cells are exposed to different environments. Normal cells may be exposed to hyperkalaemia; abnormal cells may be perfused by normal environment. For example, with a healed myocardial infarction, abnormal cells can be exposed to an abnormal environment such as with a myocardial infarction with myocardial ischaemia. In conditions such as myocardial ischaemia, possible mechanism of arrhythmia generation include the resulting decreased internal K+ concentration, the increased external K+ concentration, norepinephrine release and acidosis.[4] When myocardial cell are exposed to hyperkaliemia, the maximum diastolic potential is depolarized as a result of the alteration of Ik1 potassium current, whose intensity and direction is strictly dependent on intracellular and extracellular potassium concentrations. With Ik1 suppressed, an hyperpolarizing effect is lost and therefore there can be activation of funny current even in myocardial cells (which is normally suppressed by the hyperpolarizing effect of coexisting potassium currents). This can lead to the instauration of automaticity in ischemic tissue.
Re-entry
The role of re-entry or circus motion was demonstrated separately by Mines and Garrey.[5] Mines created a ring of excitable tissue by cutting the atria out of the ray fish. Garrey cut out a similar ring from the turtle ventricle. They were both able to show that, if a ring of excitable tissue was stimulated at a single point, the subsequent waves of depolarisation would pass around the ring. The waves eventually meet and cancel each other out, but, if an area of transient block occurred with a refractory period that blocked one wavefront and subsequently allowed the other to proceed retrogradely over the other path, then a self-sustaining circus movement phenomenon would result. For this to happen, however, it is necessary that there be some form of non-uniformity. In practice, this may be an area of ischaemic or infarcted myocardium, or underlying scar tissue.
It is possible to think of the advancing wave of depolarisation as a dipole with a head and a tail. The length of the refractory period and the time taken for the dipole to travel a certain distance—the propagation velocity—will determine whether such a circumstance will arise for re-entry to occur. Factors that promote re-entry would include a slow-propagation velocity, a short refractory period with a sufficient size of ring of conduction tissue. These would enable a dipole to reach an area that had been refractory and is now able to be depolarised with continuation of the wavefront.
In clinical practice, therefore, factors that would lead to the right conditions to favour such re-entry mechanisms include increased heart size through hypertrophy or dilatation, drugs which alter the length of the refractory period and areas of cardiac disease. Therefore, the substrate of ventricular fibrillation is transient or permanent conduction block. Block due either to areas of damaged or refractory tissue leads to areas of myocardium for initiation and perpetuation of fibrillation through the phenomenon of re-entry.
Pathophysiology
Ventricular fibrillation has been described as "chaotic asynchronous fractionated activity of the heart" (Moe et al. 1964). A more complete definition is that ventricular fibrillation is a "turbulent, disorganized electrical activity of the heart in such a way that the recorded electrocardiographic deflections continuously change in shape, magnitude and direction".[6]
Ventricular fibrillation most commonly occurs within diseased hearts, and, in the vast majority of cases, is a manifestation of underlying ischemic heart disease. Ventricular fibrillation is also seen in those with cardiomyopathy, myocarditis, and other heart pathologies. In addition, it is seen with electrolyte disturbances and overdoses of cardiotoxic drugs. It is also notable that ventricular fibrillation occurs where there is no discernible heart pathology or other evident cause, the so-called idiopathic ventricular fibrillation.
Idiopathic ventricular fibrillation occurs with a reputed incidence of approximately 1% of all cases of out-of-hospital arrest, as well as 3%-9% of the cases of ventricular fibrillation unrelated to myocardial infarction, and 14% of all ventricular fibrillation resuscitations in patients under the age of 40.[7] It follows then that, on the basis of the fact that ventricular fibrillation itself is common, idiopathic ventricular fibrillation accounts for an appreciable mortality. Recently described syndromes such as the Brugada Syndrome may give clues to the underlying mechanism of ventricular arrhythmias. In the Brugada syndrome, changes may be found in the resting ECG with evidence of right bundle branch block (RBBB) and ST elevation in the chest leads V1-V3, with an underlying propensity to sudden cardiac death.[8]
The relevance of this is that theories of the underlying pathophysiology and electrophysiology must account for the occurrence of fibrillation in the apparent "healthy" heart. It is evident that there are mechanisms at work that we do not fully appreciate and understand. Investigators are exploring new techniques of detecting and understanding the underlying mechanisms of sudden cardiac death in these patients without pathological evidence of underlying heart disease.[9]
Familial conditions that predispose individuals to developing ventricular fibrillation and sudden cardiac death are often the result of gene mutations that affect cellular transmembrane ion channels. For example, in Brugada Syndrome, sodium channels are affected. In certain forms of long QT syndrome, the potassium inward rectifier channel is affected.
Triggered activity
Triggered activity can occur due to the presence of afterdepolarisations. These are depolarising oscillations in the membrane voltage induced by preceding action potentials. These can occur before or after full repolarisation of the fiber and as such are termed either early (EADs) or delayed afterdepolarisations (DADs). All afterdepolarisations may not reach threshold potential, but, if they do, they can trigger another afterdepolarisation, and thus self-perpetuate.
Characteristics of the ventricular fibrillation waveform
Ventricular fibrillation can be described in terms of its electrocardiographic waveform appearance. All waveforms can be described in terms of certain features, such as amplitude and frequency. Researchers have looked at the frequency of the ventricular fibrillation waveform to see if it helps to elucidate the underlying mechanism of the arrhythmia or holds any clinically useful information. More recently, Gray has suggested an underlying mechanism for the frequency of the waveform that has puzzled investigators as possibly being a manifestation of the Doppler effect of rotors of fibrillation.[10] Analysis of the fibrillation waveform is performed using a mathematical technique known as Fourier analysis.
Power spectrum
The distribution of frequency and power of a waveform can be expressed as a power spectrum in which the contribution of different waveform frequencies to the waveform under analysis is measured. This can be expressed as either the dominant or peak frequency, i.e., the frequency with the greatest power or the median frequency, which divides the spectrum in two halves.
Frequency analysis has many other uses in medicine and in cardiology, including analysis of heart rate variability and assessment of cardiac function, as well as in imaging and acoustics.[11][12]
Histopathology
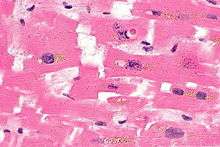
Myofibre break-up, abbreviated MFB, is associated with ventricular fibrillation leading to death.[13] Histomorphologically, MFB is characterized by fractures of the cardiac myofibres perpendicular to their long axis, with squaring of the myofibre nuclei.
Treatment
Defibrillation
Electric defibrillator
The condition can often be reversed by the electric discharge of direct current from a defibrillator. Although a defibrillator is designed to correct the problem, and its effects can be dramatic, it is not always successful.
Implantable electric defibrillator
In patients at high risk of ventricular fibrillation, the use of an implantable cardioverter defibrillator has been shown to be beneficial.
Precordial thump
If no defibrillator is available, a precordial thump can be delivered at the onset of VF for a small chance to regain cardiac function. A precordial thump may only be delivered if a cardiac arrest is witnessed (someone sees the patient arrest) and if the arrest is monitored (as seen on a cardiac monitor). However, research has shown that the precordial thump releases no more than 30 joules of energy. This is far less than the 200–360 J typically used to bring about normal sinus rhythm.
Antiarrhythmic agents
Antiarrhythmic agents like amiodarone or lidocaine can help, but, unlike atrial fibrillation, ventricular fibrillation rarely reverses spontaneously in large adult mammals. Drug therapy with antiarrhythmic agents in ventricular fibrillation does not replace defibrillation and is not the first priority, but is sometimes needed in cases where initial defibrillation attempts are not successful.
Epidemiology
Sudden cardiac arrest is the leading cause of death in the industrialised world. It exacts a significant mortality with approximately 70,000 to 90,000 sudden cardiac deaths each year in the United Kingdom, and survival rates are only 2%.[14] The majority of these deaths are due to ventricular fibrillation secondary to myocardial infarction, or "heart attack".[15] During ventricular fibrillation, cardiac output drops to zero, and, unless remedied promptly, death usually ensues within minutes.
History of knowledge of ventricular fibrillation
Lyman Brewer suggests that the first recorded account of ventricular fibrillation dates as far back as 1500 BC, and can be found in the Ebers papyrus of ancient Egypt. The extract recorded 3500 years ago may even date from as far back as 3500 BC. It states: "When the heart is diseased, its work is imperfectly performed: the vessels proceeding from the heart become inactive, so that you cannot feel them … if the heart trembles, has little power and sinks, the disease is advanced and death is near." A book authored by Jo Miles suggests that it may even go back farther. Tests done on frozen remains found in the Himalayas seemed fairly conclusive that the first known case of ventricular fibrillation dates back to at least 2500 BC.[16]
Whether this is a description of ventricular fibrillation is debatable.[17] The next recorded description occurs 3000 years later and is recorded by Vesalius, who described the appearance of "worm-like" movements of the heart in animals prior to death.
The significance and clinical importance of these observations and descriptions possibly of ventricular fibrillation were not recognised until John Erichsen in 1842 described ventricular fibrillation following the ligation of a coronary artery (Erichsen JE 1842). Subsequent to this in 1850, fibrillation was described by Ludwig and Hoffa when they demonstrated the provocation of ventricular fibrillation in an animal by applying a "Faradic" (electrical) current to the heart.[18]
In 1874, Edmé Félix Alfred Vulpian coined the term mouvement fibrillaire, a term that he seems to have used to describe both atrial and ventricular fibrillation.[19] John A. MacWilliam, a physiologist who had trained under Ludwig and who subsequently became Professor of Physiology at the University of Aberdeen, gave an accurate description of the arrhythmia in 1887. This definition still holds today, and is interesting in the fact that his studies and description predate the use of electrocardiography. His description is as follows: "The ventricular muscle is thrown into a state of irregular arrhythmic contraction, whilst there is a great fall in the arterial blood pressure, the ventricles become dilated with blood as the rapid quivering movement of their walls is insufficient to expel their contents; the muscular action partakes of the nature of a rapid incoordinate twitching of the muscular tissue … The cardiac pump is thrown out of gear, and the last of its vital energy is dissipated in the violent and the prolonged turmoil of fruitless activity in the ventricular walls." MacWilliam spent many years working on ventricular fibrillation and was one of the first to show that ventricular fibrillation could be terminated by a series of induction shocks through the heart.[20]
The first electrocardiogram recording of ventricular fibrillation was by August Hoffman in a paper published in 1912.[21] At this time, two other researchers, Mines and Garrey, working separately, produced work demonstrating the phenomenon of circus movement and re-entry as possible substrates for the generation of arrhythmias. This work was also accompanied by Lewis, who performed further outstanding work into the concept of "circus movement."
Later milestones include the work by W. J. Kerr and W. L. Bender in 1922, who produced an electrocardiogram showing ventricular tachycardia evolving into ventricular fibrillation.[22] The re-entry mechanism was also advocated by DeBoer, who showed that ventricular fibrillation could be induced in late systole with a single shock to a frog heart.[23] The concept of "R on T ectopics" was further brought out by Katz in 1928.[24] This was called the “vulnerable period” by Wiggers and Wegria in 1940, who brought to attention the concept of the danger of premature ventricular beats occurring on a T wave.
Another definition of VF was produced by Wiggers in 1940. He described ventricular fibrillation as "an incoordinate type of contraction which, despite a high metabolic rate of the myocardium, produces no useful beats. As a result, the arterial pressure falls abruptly to very low levels, and death results within six to eight minutes from anemia of the brain and spinal cord".[25]
Spontaneous conversion of ventricular fibrillation to a more benign rhythm is rare in all but small animals. Defibrillation is the process that converts ventricular fibrillation to a more benign rhythm. This is usually by application of an electric shock to the myocardium and is discussed in detail in the relevant article.
See also
- Asystole
- Atrial fibrillation
- Cardiac arrest
- Electric shock
- Flatline
- Osborn wave
- Re-entry ventricular arrhythmia
- Ventricular flutter
References
- ↑ Michael E Zevitz, MD. "Ventricular Fibrillation". Medscape. Retrieved 2011-08-17.
- ↑ "Ventricular fibrillation". U.S. National Library of Medicine.
- ↑ Weineng Wang, Zhiqiang Wang, Xiao Peng, Effects of the Earth Current Frequency and Distortion on Residual Current Devices, Scientific Journal of Control Engineering, Dec 2013, Vol 3 Issue 6 pp 417-422
- ↑ Ho K 1993
- ↑ Mines GR 1913, Garrey WE 1914
- ↑ Robles de Medina EO, Bernard R, Coumel P, et al. (1978). "Definition of terms related to cardiac rhythm. WHO/ISFC Task Force". Eur J Cardiol 8 (2): 127–44. PMID 699945.
- ↑ Viskin S, Belhassen B (1990). "Idiopathic ventricular fibrillation". Am. Heart J. 120 (3): 661–71. doi:10.1016/0002-8703(90)90025-S. PMID 2202193.
- ↑ Brugada P, Brugada J (1992). "Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report". J. Am. Coll. Cardiol. 20 (6): 1391–6. doi:10.1016/0735-1097(92)90253-J. PMID 1309182.
- ↑ Saumarez RC, Heald S, Gill J, et al. (1995). "Primary ventricular fibrillation is associated with increased paced right ventricular electrogram fractionation". Circulation 92 (9): 2565–71. doi:10.1161/01.cir.92.9.2565. PMID 7586358.
- ↑ Jalife J, Gray RA, Morley GE, Davidenko JM (1998). "Self-organization and the dynamical nature of ventricular fibrillation". Chaos 8 (1): 79–93. doi:10.1063/1.166289. PMID 12779712.
- ↑ Shusterman V, Beigel A, Shah SI, et al. (1999). "Changes in autonomic activity and ventricular repolarization". J Electrocardiol. 32. Suppl: 185–92. doi:10.1016/S0022-0736(99)90078-X. PMID 10688324.
- ↑ Kaplan SR, Bashein G, Sheehan FH, et al. (2000). "Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve". Am. Heart J. 139 (3): 378–87. doi:10.1016/S0002-8703(00)90077-2. PMID 10689248.
- ↑ Baroldi, G.; Silver, MD.; Parolini, M.; Pomara, C.; Turillazzi, E.; Fineschi, V. (Apr 2005). "Myofiberbreak-up: a marker of ventricular fibrillation in sudden cardiac death.". Int J Cardiol 100 (3): 435–41. doi:10.1016/j.ijcard.2004.10.007. PMID 15837088.
- ↑ National Institute for Health and Clinical Excellence Guidelines 2000
- ↑ Myerburg RJ et al. 1995
- ↑ Brewer LA (1983). "Sphygmology through the centuries. Historical notes". Am. J. Surg. 145 (6): 695–701. doi:10.1016/0002-9610(83)90124-1. PMID 6344674.
- ↑ Brewer LA (1983). "Sphygmology through the centuries. Historical notes". Am. J. Surg. 145 (6): 696–702. doi:10.1016/0002-9610(83)90124-1. PMID 6344674.
- ↑ Hoffa M et al. 1850
- ↑ Vulpian A 1874
- ↑ MacWilliam JA 1887
- ↑ Hoffman A 1912
- ↑ Kerr, WJ et al. 1922
- ↑ De Boer S 1923
- ↑ Katz LN 1928
- ↑ Wiggers, CJ et al. 1940
External links
- Interactive models and information on ventricular fibrillation and other arrhythmias
- Research on Primary Ventricular Fibrillation during Acute Myocardial Infarction
- When a child's heart stops, onset time of abnormal rhythms is crucial
- http://arrhythmiatube.com/untitled-resource
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||