Valsartan
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
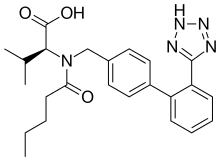
(S)-3-methyl-2-(N-{[2'-(2H-1,2,3,4-tetrazol-5-yl)biphenyl-4-yl]methyl}pentanamido)butanoic acid | |
| Clinical data | |
| Trade names | Diovan |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a697015 |
| Licence data | |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | oral |
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Protein binding | 95% |
| Biological half-life | 6 hours |
| Excretion | Renal 30%, biliary 70% |
| Identifiers | |
| CAS Number |
137862-53-4 |
| ATC code | C09CA03 |
| PubChem | CID 60846 |
| IUPHAR/BPS |
3937 tritiated: 593 |
| DrugBank |
DB00177 |
| ChemSpider |
54833 |
| UNII |
80M03YXJ7I |
| KEGG |
D00400 |
| ChEBI |
CHEBI:9927 |
| ChEMBL |
CHEMBL1069 |
| Chemical data | |
| Formula | C24H29N5O3 |
| Molar mass | 435.519 g/mol |
| |
| |
| (verify) | |
Valsartan (trade name Diovan) is an angiotensin II receptor antagonist (commonly called an ARB, or angiotensin receptor blocker), that is selective for the type I (AT1) angiotensin receptor. Valsartan is mainly used for treatment of high blood pressure, congestive heart failure, and to increase the chances of living longer after a heart attack.
Medical uses
Valsartan is used to treat high blood pressure, congestive heart failure, and to reduce death for people with left ventricular dysfunction after having had a heart attack.[1][2]
There is contradictory evidence with regard to treating people with heart failure with a combination of an angiotensin receptor blocker like valsartan and an angiotensin-converting enzyme inhibitor, with two major clinical trials (CHARM-additive and ValHeFt) showing a reduction in death, and two others (VALIANT and ONTARGET) showing no benefits, and more adverse effects including heart attacks.[1]
In people with type II diabetes and high blood pressure or albumin in the urine, valsartan is used to slow the worsening and the development end-stage renal disease.[3]
Contraindications
The packaging for valsartan includes a warning stating the drug should not be used with the renin inhibitor aliskiren in people with diabetes mellitus. It also states the drug should not be used in people with kidney disease.[2]
Valsartan falls in FDA pregnancy category D and includes a black box warning for fetal toxicity.[2] Discontinuation of these agents is recommended immediately after detection of pregnancy and an alternative medication should be started.[2] The US labeling makes no recommendation regarding continuation or discontinuation of valsartan for breast-feeding mothers.[2] The Canadian labeling does not recommend use by nursing women.[4]
Side effects
Rates of side effects depends on the reason the medication is used.
Heart failure
Rates of adverse effects are based on a comparison versus placebo in people with heart failure.[5] Most common side effects include dizziness (17% vs 9% ), low blood pressure (7% vs 2%), and diarrhea (5% vs 4%).[5] Less common side effects include joint pain, fatigue, and back pain (all 3% vs 2%).[5]
Hypertension
Clinical trials for valsartan treatment for hypertension versus placebo demonstrate side effects like viral infection (3% vs 2%), fatigue (2% vs 1%) and abdominal pain (2% vs 1%). Minor side effects that occurred at >1% but were similar to rates from the placebo group include:[5]
- headache
- dizziness
- upper respiratory infection
- cough
- diarrhea
- rhinitis/sinusitis
- nausea
- pharyngitis
- edema
- arthralgia
Interactions
%2C_Singapore_-_20150210.jpg)
The US prescribing information lists the following drug interactions for valsartan:
- Other inhibitors of the renin-angiotensin system may increase the risks of low blood pressure, kidney problems, and hyperkalemia
- Potassium sparing diuretics, potassium supplements, salt substitutes containing potassium may increase the risk of hyperkalemia.
- NSAIDs may increase the risk of kidney problems and may interfere with blood pressure-lowering effects.
- Valsartan may increase the concentration of lithium.[2]
Mechanism of action
Valsartan blocks the actions of angiotensin II, which include constricting blood vessels and activating aldosterone, to reduce blood pressure.[6] The drug binds to angiotensin type I receptors (AT1), working as an antagonist. This mechanism of action is different than the ACE inhibitor drugs, which block the conversion of angiotensin I to angiotensin II. Since valsartan acts at the receptor, it can provide more complete angiotensin II antagonism since angiotensin II is generated by other enzymes as well as ACE. Also, valsartan does not affect the metabolism of bradykinin like ACE inhibitors do.[6]
Economics
In 2010, valsartan (trade name Diovan) achieved annual sales of $2.052 billion in the United States and $6.053 billion worldwide.[7] The patents for valsartan and valsartan/hydrochlorothiazide expired in September 2012.[8][9]
Research
In people with impaired glucose tolerance, valsartan may decrease the incidence of developing diabetes mellitus type 2. However, the absolute risk reduction is small (less than 1 percent per year) and diet, exercise or other drugs, may be more protective. In the same study, no reduction in the rate of cardiovascular events (including death) was shown.[10]
A prospective study released in 2010, based on 819,491 cases in U.S. Department of Veterans Affairs database from 2002 to 2006, demonstrated a significant reduction in the incidence and progression of Alzheimer's disease and dementia.[11]
See also
- Angiotensin II receptor antagonist
- Discovery and development of angiotensin receptor blockers
- Valsartan/hydrochlorothiazide
References
- 1 2 Randa, Hilal-Dandan (2011). "Chapter 26. Renin and Angiotensin". In Brunton, L. L.; Chabner, Bruce; Knollmann, Björn C. Goodman & Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
- 1 2 3 4 5 6 "Diovan prescribing information" (PDF). Novartis.
- ↑ Inzucchi, Silvio E.; Bergenstal, Richard M.; Buse, John B.; Diamant, Michaela; Ferrannini, Ele; Nauck, Michael; Peters, Anne L.; Tsapas, Apostolos; Wender, Richard (2015-01-01). "Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes". Diabetes Care 38 (1): 140–149. doi:10.2337/dc14-2441. ISSN 0149-5992. PMID 25538310.
- ↑ "DIOVAN Product Monograph". Health Canada Drug Product Database. Novartis Pharmaceuticals Canada Inc. Retrieved 5 November 2015.
- 1 2 3 4 "DailyMed - VALSARTAN - valsartan tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-04.
- 1 2 Katzung, Bertram G; Trevor, Anthony J. (2015). "Chapter 11". Basic & Clinical Pharmacology (13 ed.). McGraw-Hill Education. ISBN 978-0071825054.
- ↑ "Novartis Annual Report 2010" (PDF).
- ↑ Philip Moeller (April 29, 2011). "Blockbuster Drugs That Will Go Generic Soon". U.S.News & World Report.
- ↑ Eva Von Schaper (August 5, 2011). "Novartis's Jimenez Has Blockbuster Plans For Diovan After Patent Expires". Bloomberg.
- ↑ McMurray JJ, Holman RR, Haffner SM, et al. (April 2010). "Effect of valsartan on the incidence of diabetes and cardiovascular events" (PDF). The New England Journal of Medicine 362 (16): 1477–90. doi:10.1056/NEJMoa1001121. PMID 20228403.
- ↑ Li NC, Lee A, Whitmer RA, et al. (January 2010). "Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis" (PDF). BMJ 340: b5465. doi:10.1136/bmj.b5465. PMC 2806632. PMID 20068258.
External links
- PubMed Valsartan
- Diovan official website Novartis
- U.S. Valsartan National Library of Medicine: Drug Information Portal
- Diovan Prescribing information Novartis
- Diovan HCT Prescribing information Novartis
| ||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||