Usher syndrome
| Usher syndrome | |
|---|---|
| Classification and external resources | |
| Specialty | ophthalmology |
| ICD-10 | H35.5 |
| OMIM | 276900 276901 |
| DiseasesDB | 13611 |
| MeSH | D052245 |
Usher syndrome is a relatively rare genetic disorder caused by a mutation in any one of at least 11 genes resulting in a combination of hearing loss and visual impairment, and is a leading cause of deafblindness. Usher syndrome is incurable at present.
Other names for Usher syndrome include Hallgren syndrome, Usher-Hallgren syndrome, retinitis pigmentosa-dysacusis syndrome, and dystrophia retinae dysacusis syndrome.[1]
Characteristics
This syndrome is characterized by hearing loss and a gradual visual impairment. The hearing loss is caused by a defective inner ear, whereas the vision loss results from retinitis pigmentosa (RP), a degeneration of the retinal cells. Usually, the rod cells of the retina are affected first, leading to early night blindness and the gradual loss of peripheral vision. In other cases, early degeneration of the cone cells in the macula occurs, leading to a loss of central acuity. In some cases, the foveal vision is spared, leading to "doughnut vision"; central and peripheral vision are intact, but an annulus exists around the central region in which vision is impaired.
Usher syndrome has three clinical subtypes, denoted as I, II, and III.[2] People with Usher I are born profoundly deaf, and begin to lose their vision in the first decade of life. They also exhibit balance difficulties, and learn to walk slowly as children, due to problems in their vestibular system. People with Usher II are not born deaf, but do have hearing loss. They do not seem to have noticeable problems with balance; they also begin to lose their vision later (in the second decade of life) and may preserve some vision even into middle age. People with Usher syndrome III are not born deaf, but experience a gradual loss of their hearing and vision; they may or may not have balance difficulties.
Usher syndrome is a variable condition; the degree of severity is not tightly linked to whether it is Usher I, II, or III. For example, someone with type III may be unaffected in childhood, but go on to develop a profound hearing loss and a very significant loss of sight by early to midadulthood. Similarly, someone with type I, who is therefore profoundly deaf from birth, may keep good central vision until the sixth decade of life, or even beyond. People with type II, who have useful hearing with a hearing aid, can experience a wide range of severity of the RP. Some may maintain good reading vision into their 60s, while others cannot see to read while still in their 40s.
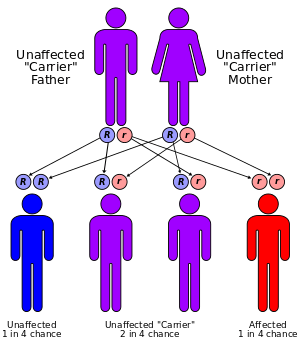
Usher syndrome I and II are associated with a mutation in any one of six or three different genes, respectively, whereas only one mutation has been linked with Usher III. Since Usher syndrome is inherited in an autosomal recessive pattern, both males and females are equally likely to inherit it. Consanguinity of the parents is a risk factor. Children of parents who both are carriers of the same mutation have a one-fourth chance of inheriting the condition, and children of such parents who are unaffected have a one-half chance of being carriers. Children of parents where only one parent is a carrier have a no chance of having the disease, but have a one-half chance of being a carrier. First recognized in the 19th century, Usher syndrome was the first condition to demonstrate that phenotypes could be inherited in tandem; deafness and blindness are inherited together, but not separately. Animal models of this human disease (such as knockout mice and zebrafish) have been developed recently to study the effects of these gene mutations and to test potential cures for Usher syndrome.
History
Usher syndrome is named after the Scottish ophthalmologist Charles Usher, who examined the pathology and transmission of this illness in 1914 on the basis of 69 cases.[3] However, it was first described in 1858 by Albrecht von Gräfe, a pioneer of modern ophthalmology.[4] He reported the case of a deaf patient with retinitis pigmentosa, who had two brothers with the same symptoms. Three years later, one of his students, Richard Liebreich, examined the population of Berlin for disease pattern of deafness with retinitis pigmentosa.[5] Liebreich noted Usher syndrome to be recessive, since the cases of blind-deafness combinations occurred particularly in the siblings of blood-related marriages or in families with patients in different generations. His observations supplied the first proofs for the coupled transmission of blindness and deafness, since no isolated cases of either could be found in the family trees.
Symptoms and subtypes
Usher syndrome is responsible for the majority of deaf-blindness.[6] The word syndrome means that multiple symptoms occur together, in this case, deafness and blindness. It occurs in roughly 1 person in 23,000 in the United States,[7] 1 in 28,000 in Norway[8] and 1 in 12,500 in Germany.[9] People with Usher syndrome represent roughly one-sixth of people with retinitis pigmentosa.[2]
Usher syndrome is inherited in an autosomal recessive pattern. "Recessive" means both parents must contribute an appropriate gene for the syndrome to appear, and "autosomal" means the gene is not carried on one of the sex chromosomes (X or Y), but rather on one of the 22 other pairs. (See the article on human genetics for more details.)
The progressive blindness of Usher syndrome results from retinitis pigmentosa.[10][11] The photoreceptor cells usually start to degenerate from the outer periphery to the center of the retina, including the macula. The degeneration is usually first noticed as night blindness (nyctalopia); peripheral vision is gradually lost, restricting the visual field (tunnel vision), which generally progresses to complete blindness. The qualifier 'pigmentosa' reflects the fact that clumps of pigment may be visible by an ophthalmoscope in advanced stages of degeneration.[2]
Although Usher syndrome has been classified clinically in several ways,[12][11][13] the prevailing approach is to classify it into three clinical sub-types called Usher I, II and III in order of decreasing severity of deafness.[10] Usher I and II are the more common forms; the fraction of people with Usher III is significant only in a few specific areas, such as Finland[14] and Birmingham.[15] As described below, these clinical subtypes may be further subdivided by the particular gene mutated; people with Usher I and II may have any one of six and three genes mutated, respectively, whereas only one gene has been associated with Usher III. The function of these genes is still poorly understood. The hearing impairment associated with Usher syndrome is better understood: damaged hair cells in the cochlea of the inner ear inhibit electrical impulses from reaching the brain.
Usher syndrome I
People with Usher I are usually born deaf and often have difficulties in maintaining their balance owing to problems in the vestibular system. Babies with Usher I are usually slow to develop motor skills such as walking. Worldwide, the estimated prevalence of Usher syndrome type I is 3 to 6 per 100,000 people in the general population.
Usher syndrome type I can be caused by mutations in any one of several different genes: CDH23, MYO7A, PCDH15, USH1C, and USH1G. These genes function in the development and maintenance of inner ear structures such as hair cells (stereocilia), which transmit sound and motion signals to the brain. Alterations in these genes can cause an inability to maintain balance (vestibular dysfunction) and hearing loss. The genes also play a role in the development and stability of the retina by influencing the structure and function of both the rod photoreceptor cells and supporting cells called the retinal pigmented epithelium. Mutations that affect the normal function of these genes can result in retinitis pigmentosa and resultant vision loss.
Type I has been found to be more common in people of Ashkenazi Jewish ancestry (central and eastern European) and in the French-Acadian populations (Louisiana).
Usher syndrome II
People with Usher II are generally hard-of-hearing rather than deaf, and their hearing does not degrade over time; moreover, they generally have a normal vestibular system. Usher syndrome type II occurs at least as frequently as type I, but because type II may be underdiagnosed or more difficult to detect, it could be up to three times as common as type I.
Usher syndrome type II may be caused by mutations in any of three different genes: USH2A, GPR98, and DFNB31. The protein encoded by the USH2A gene, usherin, is located in the supportive tissue in the inner ear and retina. Usherin is critical for the proper development and maintenance of these structures, which may help explain its role in hearing and vision loss. The location and function of the other two proteins are not yet known.
Usher syndrome III
By contrast, people with Usher III experience a 'progressive' loss of hearing and roughly half have vestibular dysfunction. The frequency of Usher syndrome type III is highest in the Finnish population, but it has been noted rarely in a few other ethnic groups.
Mutations in only one gene, CLRN1, have been linked to Usher syndrome type III. CLRN1 encodes clarin-1, a protein important for the development and maintenance of the inner ear and retina. However, the protein's function in these structures, and how its mutation causes hearing and vision loss, is still poorly understood.
Differential diagnosis
Since Usher syndrome is incurable at present, it is helpful to diagnose children well before they develop the characteristic night blindness. Some preliminary studies have suggested as many as 10% of congenitally deaf children may have Usher syndrome.[1] However, a misdiagnosis can have bad consequences.
The simplest approach to diagnosing Usher syndrome is to test for the characteristic chromosomal mutations. An alternative approach is electroretinography, although this is often disfavored for children, since its discomfort can also make the results unreliable.[1] Parental consanguinity is a significant factor in diagnosis. Usher syndrome I may be indicated if the child is profoundly deaf from birth and especially slow in walking.
Thirteen other syndromes may exhibit signs similar to Usher syndrome, including Alport syndrome, Alstrom syndrome, Bardet-Biedl syndrome, Cockayne syndrome, spondyloepiphyseal dysplasia congenita, Flynn-Aird syndrome, Friedreich ataxia, Hurler syndrome (MPS-1), Kearns-Sayre syndrome (CPEO), Norrie syndrome, osteopetrosis (Albers-Schonberg disease), Refsum's disease (phytanic acid storage disease), and Zellweger syndrome (cerebrohepatorenal syndrome).
Genes associated with Usher syndrome
| Type | Freq [16] | Gene locus | Gene | Protein | Function | Size (AA) | UniProt | OMIM |
|---|---|---|---|---|---|---|---|---|
| USH1B | 39–55% | 11q13.5 | MYO7A | Myosin VIIA | Motor protein | 2215 | Q13402 | 276900 |
| USH1C | 6–7% | 11p15.1-p14 | USH1C | Harmonin | PDZ-domain protein | 552 | Q9Y6N9 | 276904 |
| USH1D | 19–35% | 10q21-q22 | CDH23 | Cadherin 23 | Cell adhesion | 3354 | Q9H251 | 601067 |
| USH1E | rare | 21q21 | ? | ? | ? | ? | ? | 602097 |
| USH1F | 11–19% | 10q11.2-q21 | PCDH15 | Protocadherin 15 | Cell adhesion | 1955 | Q96QU1 | 602083 |
| USH1G | 7% | 17q24-q25 | USH1G | SANS | Scaffold protein | 461 | Q495M9 | 606943 |
| USH2A | 80% | 1q41 | USH2A | Usherin | Transmembrane linkage | 5202 | O75445 | 276901 |
| USH2C | 15% | 5q14.3-q21.1 | GPR98 | VLGR1b | Very large GPCR | 6307 | Q8WXG9 | 605472 |
| USH2D | 5% | 9q32-q34 | DFNB31 | Whirlin | PDZ-domain protein | 907 | Q9P202 | 611383 |
| USH3A | 100% | 3q21-q25 | CLRN1 | Clarin-1 | Synaptic shaping | 232 | P58418 | 276902 |
Several genes have been associated with Usher syndrome using linkage analysis of patient families (Table 1) and DNA sequencing of the identified loci.[17][18] A mutation in any one of these genes is likely to result in Usher syndrome. The clinical subtypes Usher I and II are associated with mutations in any one of six (USH1B-G) and three (USH2A ,C-D) genes, respectively, whereas only one gene, USH3A, has been linked to Usher III so far. Two other genes, USH1A and USH2B, were initially associated with Usher syndrome, but USH2B has not been verified and USH1A was incorrectly determined and does not exist.[19] Research in this area is ongoing.
Using interaction analysis techniques, the identified gene products could be shown to interact with one another in one or more larger protein complexes. If one of the components is missing, this protein complex cannot fulfill its function in the living cell, and it probably comes to the degeneration the same. The function of this protein complex has been suggested to participate in the signal transduction or in the cell adhesion of sensory cells.[18]
Prospects for gene therapy
Since Usher syndrome results from the loss of a gene, gene therapy that adds the proper protein back ("gene replacement") may alleviate it, provided the added protein becomes functional. Recent studies of mouse models have shown one form of the disease — that associated with a mutation in myosin VIIa — can be alleviated by replacing the mutant gene using a lentivirus.[20] However, some of the mutated genes associated with Usher syndrome encode very large proteins — most notably, the USH2A and GPR98 proteins, which have roughly 6000 amino-acid residues. Gene replacement therapy for such large proteins may be difficult.
Individual cases
A 31-year-old woman with Usher syndrome, Rebecca Alexander, was profiled in Marie Claire in November 2007.[21] After graduating from the University of Michigan with excellent marks, Alexander went on to Columbia University, where she earned two master's degrees in public health and clinical social work. Rebecca is an active member of her community, working with various charities in NYC. Rebecca's dedication as an active member of her community was most notably recognized when she was selected as a "Community Hero" to run with the Olympic Torch for the 1996 Atlanta Olympic Games in honor of her volunteer work for Project Open Hand, a nonprofit organization delivering meals to people living with HIV/AIDS in the San Francisco Bay area. Rebecca received her psychodynamic psychotherapy training through the American Institute of Psychoanalysis. She currently works in private practice specializing in the treatment of mood and anxiety disorders, eating disorders, addictions, disability, and trauma. While currently facilitating group seminars for the Foundation Fighting Blindness during national conferences, Rebecca is also in the process of launching the Usher III Initiative, a nonprofit organization dedicated to science and research that seeks to find a cure for Usher III. Rebecca teaches indoor cycling/spin classes with a strong following at select gyms in New York City. She was featured on NBC's Today Show on March 20, 2009 which has been nominated for an Emmy award in September 2010. She is the sister of NBC News National Correspondent Peter Alexander. Rebecca appeared again on the Today Show in September 2014 with her brother and discussed her experiences with the disease and her recently released book "Not Fade Away: A Memoir of Senses Lost and Found".
Christine "Coco" Roschaert ref>Coco's blog</ref> Born in Ottawa, ON on 5 January, 1980. Christine is a well-known person with Usher syndrome. She has published video blogs at YouTube,[22] and recently was the kick-off speaker for the Deaf Awareness Week at the University of Vermont.[23] In 2006, she graduated with a degree in Communication Sciences from Gallaudet University; there, she was a hunger striker in the 2006 protest organized by the Gallaudet United Now Movement.[24] Roschaert is now in Nigeria founding the first deafblind program in that country.
A web-community, UsherLife, of people with Usher syndrome was founded on 1 February 2005 by Nick Sturley. Although centered on Great Britain, it offers resources to all people with Usher syndrome. The organization is hosting regular get-togethers in England, such as the Usher Hood Pub in Nottingham[25] and a trip to Brighton pier.[26] Other people with Usher syndrome have posted videos about their lives and condition on YouTube, most notably Ginny Paja-Nyholm.[27] In October 2007, Candice, a mom living in Texas, began blogging about her two daughters, Jasmine and Rebecca; Rebecca has Usher syndrome I.[28]
Catherine Fischer has written a well-received autobiography of growing up with Usher syndrome in Louisiana, entitled Orchid of the Bayou.[29] Similarly, Vendon Wright has written two books describing his life with Usher syndrome, I was blind but now I can see[30] and Through my eyes.[31] Louise Boardman has also written a short book called My son has Usher's Syndrome.[32]
Christian Markovic,ls an artist living with Usher syndrome, runs a company, Fuzzy Wuzzy Designs.[33]
Spencer Tracy's son John was a well-known person with Usher syndrome who lived a full life.[34] The John Tracy Clinic was founded in 1942 by his mother Louise to offer free help to parents of hearing-impaired infants and preschool children.[1]
Jacob Desormeaux, son of horse-racing jockey Kent Desormeaux, has Usher syndrome. Jacob was born deaf and is progressively going blind. Kent dedicated his race in the Belmont Stakes, which would give him and his horse Big Brown the Triple Crown, to his son Jacob. The family has started an organization to raise funds and awareness of the disease. Usher syndrome is disproportionately common among the Cajuns of south Louisiana, such as Desormeaux and Fischer, because of a genetic mutation among early French Acadian settlers in Nova Scotia.
DNA helix co-discoverer and Nobel laureate James D. Watson has homozygous USH1B mutations, according to his published genome.[35] It is not clear why he did not develop the syndrome. This lack of genetic penetrance argues that expression of the phenotype of Usher syndrome may be more complex than originally assumed.
The Israeli Nalaga'at (do touch) Deaf-blind Acting Ensemble consists of 11 deaf-blind actors, most of whom are diagnosed with Usher syndrome. The theater group has put on several productions and appeared both locally in Israel and abroad in London and Broadway.[36]
References
- 1 2 3 4 Mets MB, Young NM, Pass A, Lasky JB (2000). "Early diagnosis of Usher syndrome in children". Transactions of the American Ophthalmological Society 98: 237–45. PMC 1298229. PMID 11190026.
- 1 2 3 Williams DS (2007). "Usher syndrome: Animal models, retinal function of Usher proteins, and prospects for gene therapy". Vision Research xx (3): xx–xx. doi:10.1016/j.visres.2007.08.015. PMC 2680226. PMID 17936325.
- ↑ Usher C (1914). "On the inheritance of Retinitis pigmentosa with notes of cases". Roy. Lond. Ophthalmol. Hosp. Rep. 19: 130–236.
- ↑ von Gräfe A (1858). "Exceptionelles Verhalten des Gesichtsfeldes bei Pigmententartung der Netzhaut". Archiv für Ophthalmologie 4: 250–253.
- ↑ Liebreich R (1861). "Abkunft aus Ehen unter Blutsverwandten als Grund von Retinitis pigmentosa". Dtsch. Klin. 13: 53.
- ↑ Vernon M (1969). "Usher's syndrome — deafness and progressive blindness. Clinical cases, prevention, theory and literature survey". Journal of Chronic Diseases 22 (3): 133–151. doi:10.1016/0021-9681(69)90055-1. PMID 4897966.
- ↑ Boughman J, Vernon M, Shaver K (1983). "Usher syndrome: Definition and estimate of prevalence from two high-risk populations". Journal of Chronic Diseases 36 (8): 595–603. doi:10.1016/0021-9681(83)90147-9. PMID 6885960.
- ↑ Grøndahl J (1987). "Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway". Clin. Genet. 31 (4): 255–264. doi:10.1111/j.1399-0004.1987.tb02804.x. PMID 3594933.
- ↑ Otterstedde CR, Spandau U, Blankenagel A, Kimberling WJ, Reisser C (2001). "A new clinical classication for Usher's syndrome based on a new subtype of Usher's syndrome type I". Laryngoscope 111 (1): 84–86. doi:10.1097/00005537-200101000-00014. PMID 11192904.
- 1 2 Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, et al. (1994). "Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium". American Journal of Medical Genetics 50 (1): 32–38. doi:10.1002/ajmg.1320500107. PMID 8160750.
- 1 2 Fishman GA, Kumar A, Joseph ME, Torok N, and Andersonj RJ (1983). "Usher's syndrome". Archives of Ophthalmology 101 (9): 1367–1374. doi:10.1001/archopht.1983.01040020369005. PMID 6604514.
- ↑ Hammerschlag V (1907). "Zur Kenntnis der hereditaer-degenerativen Taubstummen und ihre differential diagnostische Bedeutung". Z. Ohrenheilk. 54: 18–36.
Bell J (1933). Retinitis Pigmentosa and Allied Diseases (2nd ed.). London: Cambridge University Press.
Hallgren B (1959). "Retinitis pigmentosa combined with congenital deafness with vestibulo-cerebellar ataxia and mental abnormality in a proportion of cases: Clinical and geneto-statistical survey". Acta Psychiatrica Scandinavica Suppl. 34 (138): 9–101. doi:10.1111/j.1600-0447.1959.tb08605.x. PMID 14399116.
Merin S, Auerbach E (1976). "Retinitis pigmentosa". Surv. Ophthalmol. 20 (5): 303–345. doi:10.1016/S0039-6257(96)90001-6. PMID 817406.
Davenport S, Omenn G (1977). The Heterogeneity of Usher Syndrome (volume 426 ed.). Amsterdam: Excerpta Medica Foundation.
Gorlin R, Tilsner T, Feinstein S, Duvall AJ (1979). "Usher syndrome type III". Arch. Otolaryngol. 105 (6): 353–354. doi:10.1001/archotol.1979.00790180051011. PMID 454290. - ↑ Sankila EM, Pakarinen H, Kääriäinen H, Aittomäki K, Karjalainen S, Sistonen P, de la Chapelle A (1995). "Assignment of Usher syndrome type III (USH3) gene to chromosome 3q". Hum. Mol. Genetics 4 (1): 93–98. doi:10.1093/hmg/4.1.93. PMID 7711740.
- ↑ Pakarinen L, Tuppurainen K, Laipapala P, Mäntyjärvi M, Puhakka H (1996). "The ophthalmological course of Usher syndrome type III". International Ophthalmology 19 (5): 307–311. doi:10.1007/BF00130927. PMID 8864816.
- ↑ Hope CI, Bundey S, Proops D, Fielder AR (1997). "Usher syndrome in the city of Birmingham — prevalence and clinical classification". British Journal of Ophthalmology 81 (1): 46–53. doi:10.1136/bjo.81.1.46. PMC 1721995. PMID 9135408.
- ↑ Roux AF, Faugere V, Le Guedard S, Pallares-Ruiz N, Vielle A, Chambert S, Marlin S, Hamel C, Gilbert B, Malcolm S, Claustres M (2006). "Survey of the frequency of USH1 gene mutations in a cohort of Usher patients shows the importance of cadherin 23 and protocadherin 15 genes and establishes a detection rate of above 90%". J Med Genet 43 (9): 763–768. doi:10.1136/jmg.2006.041954. PMC 2564578. PMID 16679490.
Ouyang XM, Yan D, Du LL, Hejtmancik JF, Jacobson SG, Nance WE, Li AR, Angeli S, Kaiser M, Newton V, Brown SD, Balkany T, Liu XZ (2005). "Characterization of Usher syndrome type I gene mutations in an Usher syndrome patient population". Hum Genet 116 (4): 292–299. doi:10.1007/s00439-004-1227-2. PMID 15660226. - ↑ Petit, C (2001). "Usher syndrome: from genetics to pathogenesis". Annual review of genomics and human genetics 2: 271–97. doi:10.1146/annurev.genom.2.1.271. PMID 11701652.
- 1 2 Reiners, J; Nagel-Wolfrum, K; Jürgens, K; Märker, T; Wolfrum, U (2006). "Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease". Experimental eye research 83 (1): 97–119. doi:10.1016/j.exer.2005.11.010. PMID 16545802.
- ↑ Gerber, S; Bonneau, D; Gilbert, B; Munnich, A; Dufier, JL; Rozet, JM; Kaplan, J (2006). "USH1A: chronicle of a slow death". American Journal of Human Genetics 78 (2): 357–9. doi:10.1086/500275. PMC 1380243. PMID 16400615.
- ↑ Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, Yang XJ, Williams DS (2007). "Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B". Gene Therapy 14 (7): 584–594. doi:10.1038/sj.gt.3302897. PMID 17268537.
- ↑ Alexander R, Grossman AJ (November 2007). "out of sight, out of sound". Marie Claire 14 (11): 191–193.
- ↑ Coco's user page at YouTube
- ↑ ASL at UVM
- ↑ First-person account of the 2006 protests at Gallaudet University
- ↑ First Usher Hood Pub on 21 July 2007 (YouTube)
- ↑ Trip to Brighton Pier on 9 June 2007 (YouTube)
- ↑ Ginny's trivia challenge for Usher syndrome
- ↑ Candice. "I'm adopting a Deaf child with Ushers, now what?". Retrieved 2007-11-07.
- ↑ Carroll C, Fischer CH (2001). Orchid of the Bayou: A Deaf Woman Faces Blindness. Gallaudet University Press. ISBN 978-1-56368-104-2.
- ↑ Wright V (2007). I was blind but now I can see. Authorhouse. ISBN 978-1-4208-9101-0.
- ↑ Wright V (2007). Through my eyes. Pipers' Ash Ltd. ISBN 978-1-904494-86-7.
- ↑ Boardman LS (1985). My son has Usher's Syndrome. Foundation Fighting Blindness. B0007207IG.
- ↑ Profile of Christian Markovic of Fuzzy Wuzzy Designs
- ↑ Associated Press obituary for John Tracy
- ↑ New England Journal of Medicine article on genomic privacy.
- ↑ http://www.nalagaat.org.il/home.php
Additional reading
- Stiefel SH, Lewis RA (1991). The Madness of Usher's: Coping With Vision and Hearing Loss/Usher Syndrome Type II. Business of Living Publications. ISBN 978-1-879518-06-3.
- Duncan E, Prickett HT (1988). Usher's Syndrome: What It Is, How to Cope, and How to Help. Charles C. Thomas. ISBN 978-0-398-05481-6.
- Vernon M (1986). Answers to your questions about Usher's syndrome (retinitis pigmentosa with hearing loss). Foundation Fighting Blindness. ASIN B00071QLJ6.
- Vernon M (1969). Usher's syndrome: Deafness and progressive blindness : clinical cases, prevention, theory and literature survey. Pergamon Press. ASIN B0007JHOJ4.
External links
General information
- GeneReviews/NCBI/NIH/UW entry on Usher Syndrome Type I
- GeneReviews/NCBI/NIH/UW entry on Usher Syndrome Type II
- Usher syndrome in the Swedish Rare Disease Database
- General overview from the NIH
- Usher Syndrome Information from the National Institute on Deafness and Other Communication Disorders (NIDCD).
- Usher syndrome Information from Sense, a British organization for deafblind people
- Scientific review of Usher 1
- Scientific review of Usher 2
- Usher Syndrome Selected Topics - National Consortium On Deaf-Blindness
Resources
- Usher Syndrome Resource Guide from the National Eye Institute (NEI).
- Usher syndrome mailing list
- Boys Town National Research Hospital
- The Williams retinal Physiology Research Group, UCSD
- Hear See Hope.com
- http://usher-syndrome.org - The Coalition for Usher Syndrome Research
- Usher III Initiative
Mutations
| ||||||||||||||||||||||||||||||||||