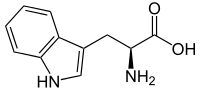
Tryptophan
 | |
| Names | |
|---|---|
| IUPAC name
Tryptophan or (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | |
| Other names
2-Amino-3-(1H-indol-3-yl)propanoic acid | |
| Identifiers | |
| 73-22-3 | |
| ChEBI | CHEBI:27897 |
| ChEMBL | ChEMBL54976 |
| ChemSpider | 6066 |
| DrugBank | DB00150 |
| 717 | |
| Jmol interactive 3D | Image |
| KEGG | D00020 |
| PubChem | 6305 |
| UNII | 8DUH1N11BX |
| |
| |
| Properties | |
| C11H12N2O2 | |
| Molar mass | 204.23 g·mol−1 |
| Soluble: 0.23 g/L at 0 °C, 11.4 g/L at 25 °C, | |
| Solubility | Soluble in hot alcohol, alkali hydroxides; insoluble in chloroform. |
| Acidity (pKa) | 2.38 (carboxyl), 9.39 (amino)[1] |
| Pharmacology | |
| ATC code | N06 |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| | |
| Infobox references | |
Tryptophan (abbreviated as Trp or W; encoded by the codon UGG) is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –+NH3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO- form under biological conditions), and a side chain indole, classifying it as a non-polar, aromatic amino acid. It is essential in humans, meaning the body cannot synthesize it and thus it must be obtained from the diet.
Tryptophan is also a precursor to neurotransmitters serotonin and melatonin.[2]
Isolation
The isolation of tryptophan was first reported by Frederick Hopkins in 1901[3] through hydrolysis of casein. From 600 grams of crude casein one obtains 4-8 grams of tryptophan.[4]
Biosynthesis and industrial production
As an essential amino acid, tryptophan is not synthesized de novo in humans and other animals, who must ingest tryptophan or tryptophan-containing proteins. Plants and microorganisms commonly synthesize tryptophan from shikimic acid or anthranilate.[5] The latter condenses with phosphoribosylpyrophosphate (PRPP), generating pyrophosphate as a by-product. After ring opening of the ribose moiety and following reductive decarboxylation, indole-3-glycerinephosphate is produced, which in turn is transformed into indole. In the last step, tryptophan synthase catalyzes the formation of tryptophan from indole and the amino acid serine.
The industrial production of tryptophan is also biosynthetic and is based on the fermentation of serine and indole using either wild-type or genetically modified bacteria such as B. amyloliquefaciens, B. subtilis, C. glutamicum or E. coli. These strains carry either mutations that prevent the reuptake of aromatic amino acids or multiple/overexpressed trp operons. The conversion is catalyzed by the enzyme tryptophan synthase.[6][7][8]
Function

For many organisms (including humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism, it is needed to prevent illness or death, and it therefore must be part of their diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis, and proteins are required to sustain life. In addition, tryptophan functions as a biochemical precursor for the following compounds (see also figure to the right):
- Serotonin (a neurotransmitter), synthesized via tryptophan hydroxylase.[9][10] Serotonin, in turn, can be converted to melatonin (a neurohormone), via N-acetyltransferase and 5-hydroxyindole-O-methyltransferase activities.[11]
- Niacin, a vitamin, is synthesized from tryptophan via kynurenine and quinolinic acids as key biosynthetic intermediates.[12]
- Auxin (a phytohormone), is mainly synthesized from tryptophan via indole-3-pyruvic acid.[13]
The disorder fructose malabsorption causes improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood,[14] and depression.[15] The authors did not find reduced tryptophan in cases of lactose maldigestion.[14]
In bacteria that synthesize tryptophan, high cellular levels of this amino acid activate a repressor protein, which binds to the trp operon.[16] Binding of this repressor to the tryptophan operon prevents transcription of downstream DNA that codes for the enzymes involved in the biosynthesis of tryptophan. So high levels of tryptophan prevent tryptophan synthesis through a negative feedback loop and, when the cell's tryptophan levels are reduced, transcription from the trp operon resumes. The genetic organisation of the trp operon thus permits tightly regulated and rapid responses to changes in the cell's internal and external tryptophan levels.
Dietary sources
Tryptophan is a routine constituent of most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, almonds, sunflower seeds, pumpkin seeds, spirulina, bananas, and peanuts. Contrary to the popular belief[17][18][19] that turkey has a particularly high amount of tryptophan, the amount of tryptophan in turkey is typical of most poultry.[20]
| Food | Tryptophan [g/100 g of food] |
Protein [g/100 g of food] |
Tryptophan/Protein [%] |
|---|---|---|---|
| egg white, dried | | | |
| spirulina, dried | | | |
| cod, atlantic, dried | | | |
| soybeans, raw | | | |
| cheese, Parmesan | | | |
| sesame seed | | | |
| cheese, cheddar | | | |
| sunflower seed | | | |
| pork, chop | | | |
| turkey | | | |
| chicken | | | |
| beef | | | |
| oats | | | |
| salmon | | | |
| lamb, chop | | | |
| perch, Atlantic | | | |
| chickpeas, raw | | | |
| egg | | | |
| wheat flour, white | | | |
| baking chocolate, unsweetened | | | |
| milk | | | |
| rice, white, medium-grain, cooked | | | |
| quinoa, uncooked | | | |
| quinoa, cooked | | | |
| potatoes, russet | | | |
| tamarind | | | |
| banana | | | |
Turkey meat and drowsiness
A common assertion in the US is that heavy consumption of turkey meat results in drowsiness, due to high levels of tryptophan contained in turkey.[17][19] However, the amount of tryptophan in turkey is comparable to that contained in most other meats.[18][20] Furthermore, post-meal drowsiness may have more to do with what is consumed along with the turkey, carbohydrates in particular.[22] It has been demonstrated in both animal models[23] and humans[24][25][26] that ingestion of a meal rich in carbohydrates triggers release of insulin. Insulin in turn stimulates the uptake of large neutral branched-chain amino acids (BCAA), but not tryptophan (an aromatic amino acid) into muscle, increasing the ratio of tryptophan to BCAA in the blood stream. The resulting increased tryptophan ratio reduces competition at the large neutral amino acid transporter (which transports both BCAA and aromatic amino acids), resulting in more uptake of tryptophan across the blood–brain barrier into the cerebrospinal fluid (CSF).[27][28] Once in the CSF, tryptophan is converted into serotonin in the raphe nuclei by the normal enzymatic pathway.[23][25] The resultant serotonin is further metabolised into melatonin by the pineal gland.[11] Hence, this data suggests that "feast-induced drowsiness"—or postprandial somnolence—may be the result of a heavy meal rich in carbohydrates, which indirectly increases the production of sleep-promoting melatonin in the brain.[23][24][25][26]
Use as a dietary supplement
Tryptophan is sold over the counter in the United States, Canada, and the United Kingdom as a dietary supplement for use as an antidepressant, anxiolytic, and sleep aid. It is also marketed as a prescription drug in some European countries for the indication of major depression under various trade names.
Since tryptophan is converted into 5-hydroxytryptophan (5-HTP) which is subsequently converted into the neurotransmitter serotonin, it has been proposed that consumption of tryptophan or 5-HTP may therefore improve depression symptoms by increasing the level of serotonin in the brain. In 2001 a Cochrane Review of the effect of 5-HTP and tryptophan on depression was published. The authors included only studies of a high rigor and included both 5-HTP and tryptophan in their review because of the limited data on either. Of 108 studies of 5-HTP and tryptophan on depression published between 1966 and 2000, only two met the authors' quality standards for inclusion, totaling 64 study participants. The substances were more effective than placebo in the two studies included but the authors state that, "the evidence was of insufficient quality to be conclusive," and note, "because alternative antidepressants exist which have been proven to be effective and safe, the clinical usefulness of 5-HTP and tryptophan is limited at present."[29] The use of tryptophan as an adjunctive therapy in addition to standard treatment for mood and anxiety disorders is not supported by the scientific evidence.[30] Due to the lack of high quality studies and preliminary nature of studies showing effectiveness and the lack of adequate study on their safety, the use of tryptophan and 5-HTP is not highly recommended or thought to be clinically useful.[29][30]
There is evidence that blood tryptophan levels are unlikely to be altered by changing the diet,[31] but tryptophan is available in health food stores as a dietary supplement.[32] Consuming purified tryptophan increases brain serotonin whereas eating foods containing tryptophan does not.[33] This is because the transport system which brings tryptophan across the blood-brain barrier is also selective for the other amino acids which are contained in protein food sources.[34] High blood plasma levels of other large neutral amino acids prevent the plasma concentration of tryptophan from increasing brain concentration levels.[34]
Side effects
Potential side effects of tryptophan include nausea, diarrhea, drowsiness, lightheadedness, headache, dry mouth, blurred vision, sedation, euphoria, and nystagmus (involuntary eye movements).[35][36] Because tryptophan has not been thoroughly studied in a clinical setting, possible side effects and interactions with other drugs are not well known.[29]
Interactions
Tryptophan has the potential to cause serotonin syndrome when combined with antidepressants of the MAOI or SSRI class or other strongly serotonergic drugs.[36]
Research
In 1912 Felix Ehrlich demonstrated that yeast attacks the natural amino acids essentially by splitting off carbon dioxide and replacing the amino group with hydroxyl. By this reaction, tryptophan gives rise to tryptophol.[37]
Tryptophan affects brain serotonin synthesis when given orally in a purified form and is used to modify serotonin levels for research in psychology.[33] Low brain serotonin is induced by administration of tryptophan-poor protein in a technique called 'acute tryptophan depletion'.[38] Studies using this method have evaluated the effect of serotonin on mood and social behavior, finding that serotonin reduces aggression and increases agreeableness.[39]
Fluorescence
Tryptophan is an important intrinsic fluorescent probe (amino acid), which can be used to estimate the nature of microenvironment of the tryptophan. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of tryptophan residues.
Safety
Eosinophilia–myalgia syndrome
There was a large outbreak of eosinophilia-myalgia syndrome (EMS) in the U.S. in 1989, with more than 1,500 cases reported to the CDC and at least 37 deaths. After preliminary investigation revealed that the outbreak was linked to intake of tryptophan, the U.S. Food and Drug Administration (FDA) banned most tryptophan from sale in the US in 1991, and other countries followed suit.[40]
Subsequent epidemiological studies suggested that EMS was linked to specific batches of L-tryptophan supplied by a single large Japanese manufacturer, Showa Denko.[40][41][42][43] It eventually became clear that recent batches of Showa Denko's L-tryptophan were contaminated by trace impurities, which were subsequently thought to be responsible for the 1989 EMS outbreak.[40][44][45] However, other evidence suggests that tryptophan itself may be a potentially major contributory factor in EMS.[46]
The FDA loosened its restrictions on sales and marketing of tryptophan in February 2001, but continued to limit the importation of tryptophan not intended for an exempted use until 2005.[40]
The fact that the Showa Denko facility used genetically engineered bacteria to produce the contaminated batches of L-tryptophan later found to have caused the outbreak of eosinophilia-myalgia syndrome has been cited as evidence of a need for "close monitoring of the chemical purity of biotechnology-derived products."[47] Those calling for purity monitoring have, in turn, been criticized as anti-GMO activists who overlook possible non-GMO causes of contamination and threaten the development of biotech.[48]
See also
- 5-HTP
- Attenuator (genetics)
- Dimethyltryptamine
- Serotonin
- Tryptamine
- Hopkins Cole reaction
- Acree-Rosenheim reaction
- Adamkiewicz reaction
References
- ↑ Dawson, RMC; et al. (1969). Data for Biochemical Research. Oxford: Clarendon Press. ISBN 0-19-855338-2.
- ↑ Slominski, Andrzej; Semak, Igor; Pisarchik, Alexander; Sweatman, Trevor; Szczesniewski, Andre; Wortsman, Jacobo. "Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells". FEBS Letters 511 (1-3): 102–106. doi:10.1016/s0014-5793(01)03319-1.
- ↑ Hopkins FG, Cole SW (Dec 1901). "A contribution to the chemistry of proteids: Part I. A preliminary study of a hitherto undescribed product of tryptic digestion". The Journal of Physiology 27 (4-5): 418–28. doi:10.1113/jphysiol.1901.sp000880. PMC 1540554. PMID 16992614.
- ↑ Cox GJ, King H (1943). "L-Tryptophane". Org. Synth 2: 612–616. doi:10.15227/orgsyn.010.0100.
- ↑ Radwanski ER, Last RL (Jul 1995). "Tryptophan biosynthesis and metabolism: biochemical and molecular genetics". The Plant Cell 7 (7): 921–34. doi:10.1105/tpc.7.7.921. PMC 160888. PMID 7640526.
- ↑ Ikeda M (2002). "Amino acid production processes". Advances in Biochemical Engineering/Biotechnology. Advances in Biochemical Engineering/Biotechnology 79: 1–35. doi:10.1007/3-540-45989-8_1. ISBN 978-3-540-43383-5. PMID 12523387.
- ↑ Becker J, Wittmann C (Aug 2012). "Bio-based production of chemicals, materials and fuels -Corynebacterium glutamicum as versatile cell factory". Current Opinion in Biotechnology 23 (4): 631–40. doi:10.1016/j.copbio.2011.11.012. PMID 22138494.
- ↑ Conrado RJ, Varner JD, DeLisa MP (Oct 2008). "Engineering the spatial organization of metabolic enzymes: mimicking nature's synergy". Current Opinion in Biotechnology 19 (5): 492–9. doi:10.1016/j.copbio.2008.07.006. PMID 18725290.
- ↑ Fernstrom JD (Apr 1983). "Role of precursor availability in control of monoamine biosynthesis in brain". Physiological Reviews 63 (2): 484–546. PMID 6132421.
- ↑ Schaechter JD, Wurtman RJ (Nov 1990). "Serotonin release varies with brain tryptophan levels" (PDF). Brain Research 532 (1-2): 203–10. doi:10.1016/0006-8993(90)91761-5. PMID 1704290.
- 1 2 Wurtman RJ, Anton-Tay F (1969). "The mammalian pineal as a neuroendocrine transducer" (PDF). Recent Progress in Hormone Research 25: 493–522. doi:10.1016/b978-0-12-571125-8.50014-4. PMID 4391290.
- ↑ Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O (Mar 1965). "Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals". The Journal of Biological Chemistry 240 (3): 1395–401. PMID 14284754.
- ↑ Palme K, Nagy F (Apr 2008). "A new gene for auxin synthesis". Cell 133 (1): 31–2. doi:10.1016/j.cell.2008.03.014. PMID 18394986.
- 1 2 Ledochowski M, Widner B, Murr C, Sperner-Unterweger B, Fuchs D (Apr 2001). "Fructose malabsorption is associated with decreased plasma tryptophan". Scandinavian Journal of Gastroenterology 36 (4): 367–71. doi:10.1080/003655201300051135. PMID 11336160.
- ↑ Ledochowski M, Sperner-Unterweger B, Widner B, Fuchs D (Jun 1998). "Fructose malabsorption is associated with early signs of mental depression". European Journal of Medical Research 3 (6): 295–8. PMID 9620891.
- ↑ Gollnick P, Babitzke P, Antson A, Yanofsky C (2005). "Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis". Annual Review of Genetics 39: 47–68. doi:10.1146/annurev.genet.39.073003.093745. PMID 16285852.
- 1 2 Helmenstine AM. "Does Eating Turkey Make You Sleepy?". About.com. Retrieved 2013-11-13.
- 1 2 Ballantyne C (2007-11-21). "Does Turkey Make You Sleepy?". Scientific American. Retrieved 2013-06-06.
- 1 2 McCue K. "Chemistry.org: Thanksgiving, Turkey, and Tryptophan". Archived from the original on 2007-04-04. Retrieved 2007-08-17.
- 1 2 3 Joanne Holden, Nutrient Data Laboratory, Agricultural Research Service. "USDA National Nutrient Database for Standard Reference, Release 22". United States Department of Agriculture. Retrieved 2009-11-29.
- ↑ Rambali B, Van Andel I, Schenk E, Wolterink G, van de Werken G, Stevenson H, Vleeming W (2002). "[The contribution of cocoa additive to cigarette smoking addiction]" (PDF). RIVM (The National Institute for Public Health and the Environment (Netherlands)) (report 650270002/2002).
- ↑ "Food & mood. (neuroscience professor Richard Wurtman) (Interview)". Nutrition Action Healthletter (HighBeam Research). September 1992.
- 1 2 3 Fernstrom JD, Wurtman RJ (Dec 1971). "Brain serotonin content: increase following ingestion of carbohydrate diet". Science 174 (4013): 1023–5. doi:10.1126/science.174.4013.1023. PMID 5120086.
- 1 2 Lyons PM, Truswell AS (Mar 1988). "Serotonin precursor influenced by type of carbohydrate meal in healthy adults" (PDF). The American Journal of Clinical Nutrition 47 (3): 433–9. PMID 3279747.
- 1 2 3 Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ (Jan 2003). "Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios". The American Journal of Clinical Nutrition 77 (1): 128–32. PMID 12499331.
- 1 2 Afaghi A, O'Connor H, Chow CM (Feb 2007). "High-glycemic-index carbohydrate meals shorten sleep onset". The American Journal of Clinical Nutrition 85 (2): 426–30. PMID 17284739.
- ↑ Pardridge WM, Oldendorf WH (Aug 1975). "Kinetic analysis of blood-brain barrier transport of amino acids". Biochimica Et Biophysica Acta 401 (1): 128–36. doi:10.1016/0005-2736(75)90347-8. PMID 1148286.
- ↑ Maher TJ, Glaeser BS, Wurtman RJ (May 1984). "Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake". The American Journal of Clinical Nutrition 39 (5): 722–9. PMID 6538743.
- 1 2 3 Shaw K, Turner J, Del Mar C (2002). Shaw KA, ed. "Tryptophan and 5-hydroxytryptophan for depression". The Cochrane Database of Systematic Reviews (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
- 1 2 Ravindran AV, da Silva TL (Sep 2013). "Complementary and alternative therapies as add-on to pharmacotherapy for mood and anxiety disorders: a systematic review". Journal of Affective Disorders 150 (3): 707–19. doi:10.1016/j.jad.2013.05.042. PMID 23769610.
- ↑ Soh NL, Walter GT (2011). "Tryptophan and depression: can diet alone be the answer?". Acta Neuropsychiatrica VL 23 (1): 1601–5215;. doi:10.1111/j.1601-5215.2010.00508.x.
- ↑ Fernstrom JD (Dec 2012). "Effects and side effects associated with the non-nutritional use of tryptophan by humans". The Journal of Nutrition 142 (12): 2236S–2244S. doi:10.3945/jn.111.157065. PMID 23077193.
- 1 2 Wurtman RJ, Hefti F, Melamed E (Dec 1980). "Precursor control of neurotransmitter synthesis". Pharmacological Reviews 32 (4): 315–35. PMID 6115400.
- 1 2 Henderson HE, Devlin R, Peterson J, Brunzell JD, Hayden MR (Dec 1990). "Frameshift mutation in exon 3 of the lipoprotein lipase gene causes a premature stop codon and lipoprotein lipase deficiency". Molecular Biology & Medicine 7 (6): 511–7. PMC 2077351. PMID 18043762.
- ↑ Kimura T, Bier DM, Taylor CL (Dec 2012). "Summary of workshop discussions on establishing upper limits for amino acids with specific attention to available data for the essential amino acids leucine and tryptophan". The Journal of Nutrition 142 (12): 2245S–2248S. doi:10.3945/jn.112.160846. PMID 23077196.
- 1 2 Howland RH (Jun 2012). "Dietary supplement drug therapies for depression". Journal of Psychosocial Nursing and Mental Health Services 50 (6): 13–6. doi:10.3928/02793695-20120508-06. PMID 22589230.
- ↑ Jackson RW (1930). "A synthesis of tryptophol" (PDF). Journal of Biological Chemistry 88 (3): 659–662.
- ↑ Young SN (Sep 2013). "Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects". Journal of Psychiatry & Neuroscience 38 (5): 294–305. doi:10.1503/jpn.120209. PMC 3756112. PMID 23428157.
- ↑ Young SN (2013). "The effect of raising and lowering tryptophan levels on human mood and social behaviour". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368 (1615): 20110375. doi:10.1098/rstb.2011.0375. PMC 3638380. PMID 23440461.
- 1 2 3 4 "Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan". FU. S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling, and Dietary Supplements. 2001-02-01. Archived from the original on 2005-02-25. Retrieved 2012-02-08.
- ↑ Slutsker L, Hoesly FC, Miller L, Williams LP, Watson JC, Fleming DW (Jul 1990). "Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer". JAMA 264 (2): 213–7. doi:10.1001/jama.264.2.213. PMID 2355442.
- ↑ Back EE, Henning KJ, Kallenbach LR, Brix KA, Gunn RA, Melius JM (Apr 1993). "Risk factors for developing eosinophilia myalgia syndrome among L-tryptophan users in New York". The Journal of Rheumatology 20 (4): 666–72. PMID 8496862.
- ↑ Kilbourne EM, Philen RM, Kamb ML, Falk H (Oct 1996). "Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome". The Journal of Rheumatology. Supplement 46: 81–8; discussion 89–91. PMID 8895184.
- ↑ Mayeno AN, Lin F, Foote CS, Loegering DA, Ames MM, Hedberg CW, Gleich GJ (Dec 1990). "Characterization of "peak E," a novel amino acid associated with eosinophilia-myalgia syndrome". Science 250 (4988): 1707–8. doi:10.1126/science.2270484. PMID 2270484.
- ↑ Ito J, Hosaki Y, Torigoe Y, Sakimoto K (Jan 1992). "Identification of substances formed by decomposition of peak E substance in tryptophan". Food and Chemical Toxicology 30 (1): 71–81. doi:10.1016/0278-6915(92)90139-C. PMID 1544609.
- ↑ Smith MJ, Garrett RH (Nov 2005). "A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation". Inflammation Research 54 (11): 435–50. doi:10.1007/s00011-005-1380-7. PMID 16307217.
- ↑ Mayeno AN, Gleich GJ (Sep 1994). "Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale". Trends in Biotechnology 12 (9): 346–52. doi:10.1016/0167-7799(94)90035-3. PMID 7765187.
- ↑ Raphals P (Nov 1990). "Does medical mystery threaten biotech?". Science 250 (4981): 619. doi:10.1126/science.2237411. PMID 2237411.
External links
- Tryptophan MS Spectrum
- "KEGG PATHWAY: Tryptophan metabolism - Homo sapiens". KEGG: Kyoto Encyclopedia of Genes and Genomes. 2006-08-23. Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (early stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (later stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Retrieved 2008-04-20.
- B Mikkelson, DP Mikkelson (2007-11-22). "Turkey Causes Sleepiness". Urban Legends Reference Pages. Snopes.com. Retrieved 2008-04-20.
- Wood RM, Rilling JK, Sanfey AG, Bhagwagar Z, Rogers RD (May 2006). "Effects of tryptophan depletion on the performance of an iterated Prisoner's Dilemma game in healthy adults". Neuropsychopharmacology 31 (5): 1075–84. doi:10.1038/sj.npp.1300932. PMID 16407905.
- Ron Sturtz (2009). "what is the difference between L-Tryptophan and 5-HTP?". The Lidtke letter: 1.
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||
|
.svg.png)
