Trimetrexate
 | |
| Systematic (IUPAC) name | |
|---|---|
|
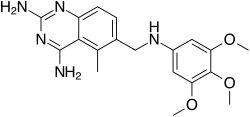
5-methyl-6-[(3,4,5-trimethoxyphenyl) aminomethyl] quinazoline-2,4-diamine | |
| Clinical data | |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a694019 |
| Pharmacokinetic data | |
| Bioavailability | VD: 20-30 Liters |
| Metabolism | Oxidative O-demethylation, followed by conjugation with glucuronide or sulfate |
| Biological half-life | 11 to 12 hours |
| Identifiers | |
| CAS Number |
52128-35-5 |
| ATC code | P01AX07 |
| PubChem | CID 5583 |
| IUPHAR/BPS | 7613 |
| DrugBank |
DB01157 |
| ChemSpider |
5381 |
| UNII |
UPN4ITI8T4 |
| KEGG |
D06238 |
| ChEMBL |
CHEMBL119 |
| Chemical data | |
| Formula | C19H23N5O3 |
| Molar mass | 369.418 g/mol |
| |
| |
| (verify) | |
Trimetrexate is a quinazoline derivative. It is a dihydrofolate reductase inhibitor.[1]
Uses
It has been used with leucovorin in treating pneumocystis pneumonia.[2]
It has been investigated for use in treating leiomyosarcoma.[3] It is a methotrexate (MTX) analog that is active against transport-deficient MTX-resistant tumor cells that overcome the acquired and natural resistance to methotrexate. Other uses include skin lymphoma. [4]
References
- ↑ Wong BK, Woolf TF, Chang T, Whitfield LR (1990). "Metabolic disposition of trimetrexate, a nonclassical dihydrofolate reductase inhibitor, in rat and dog". Drug Metab. Dispos. 18 (6): 980–6. PMID 1981548.
- ↑ Sattler FR, Allegra CJ, Verdegem TD, et al. (January 1990). "Trimetrexate-leucovorin dosage evaluation study for treatment of Pneumocystis carinii pneumonia". J. Infect. Dis. 161 (1): 91–6. doi:10.1093/infdis/161.1.91. PMID 2136905.
- ↑ Smith HO, Blessing JA, Vaccarello L (January 2002). "Trimetrexate in the treatment of recurrent or advanced leiomyosarcoma of the uterus: a phase II study of the Gynecologic Oncology Group". Gynecol. Oncol. 84 (1): 140–4. doi:10.1006/gyno.2001.6482. PMID 11748990.
- ↑ Trimetrexate in relapsed T-cell lymphoma with skin involvement. J Clin Oncol. 2002 Jun 15;20(12):2876-80.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Wednesday, September 02, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.