Trifluoromethanesulfonic acid
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Trifluoromethanesulfonic acid | |||
| Other names
Triflic acid | |||
| Identifiers | |||
| 1493-13-6 | |||
| ChEBI | CHEBI:48511 | ||
| ChemSpider | 56192 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 62406 | ||
| |||
| |||
| Properties | |||
| CF3SO3H | |||
| Molar mass | 150.08 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.696 g/mL | ||
| Melting point | −40 °C (−40 °F; 233 K) | ||
| Boiling point | 162 °C (324 °F; 435 K) | ||
| Miscible | |||
| Hazards | |||
| Main hazards | Corrosive, eye irritant | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.[1][2] It is a hygroscopic, colorless, slightly viscous liquid which is soluble in polar solvents.
Synthesis
Trifluoromethanesulfonic acid is produced industrially by electrochemical fluorination (ECF) of methanesulfonic acid:
- CH3SO3H + 4 HF → CF3SO2F + H2O + 1.5 H2
The resulting CF3SO2F is hydrolyzed, and the resulting triflate salt is preprotonated. Alternatively, trifluoromethanesulfonic acid arises by oxidation of trifluoromethylsulfenyl chloride:[3]
- CF3SCl + 2 Cl2 + 2 H2O → CF3SO2OH + 4 HCl
Triflic acid is purified by distillation from triflic anhydride.[2]
Historical
Trifluoromethanesulfonic acid was first synthesized in 1954 by Haszeldine and Kidd by the following reaction:[4]
Reactions
As an acid
In the laboratory, triflic acid is useful in protonations because the conjugate base of triflic acid is nonnucleophilic. It is also used as an acidic titrant in nonaqueous acid-base titration because it behaves as a strong acid in many solvents (acetonitrile, acetic acid, etc.) where common mineral acids (such as HCl or H2SO4) are only moderately strong.
With a Ka = 1 ×1012 (pKa ~ −12)[5] Triflic acid qualifies as a superacid. It owes many of its useful properties to its great thermal and chemical stability. Both the acid and its conjugate base CF3SO−
3, known as triflate, resist oxidation/reduction reactions, whereas many strong acids are oxidizing, e.g. HClO4 and HNO3. Further recommending its use, triflic acid does not sulfonate substrates, which can be a problem with sulfuric acid, fluorosulfuric acid, and chlorosulfonic acid. Below is a prototypical sulfonation, which HOTf does not undergo:
- C6H6 + H2SO4 → C6H5(SO3H) + H2O
Triflic acid fumes in moist air and forms a stable solid monohydrate, CF3SO3H·H2O, melting point 34 °C.
Salt and complex formation
The triflate ligand is labile, reflecting its low basicity. Trifluoromethanesulfonic acid exothermically reacts with metal carbonates, hydroxides, and oxides. Illustrative is the synthesis of Cu(OTf)2.[6]
- CuCO3 + 2 CF3SO3H → Cu(O3SCF3)2 + H2O + CO2
Chloride ligands can be converted to the corresponding triflates:
- 3 CF3SO3H + [Co(NH3)5Cl]Cl2 → [Co(NH3)5O3SCF3](O3SCF3)2 + 3 HCl
This conversion is conducted in neat HOTf at 100 °C, followed by precipitation of the salt upon the addition of ether.
Organic chemistry
Triflic acid reacts with acyl halides to give mixed triflate anhydrides, which are strong acylating agents, e.g. in Friedel-Crafts reactions.
- CH3C(O)Cl + CF3SO3H → CH3C(O)OSO2CF3 + HCl
- CH3C(O)OSO2CF3 + C6H6 → CH3C(O)C6H5 + CF3SO3H
Triflic acid catalyzes the reaction of aromatic compounds with sulfonyl chlorides, probably also via the intermediacy of a mixed anhydride of the sulfonic acid.
Triflic acid promotes other Friedel-Crafts-like reactions including the cracking of alkanes and alkylation of alkenes, which are very important to the petroleum industry. These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum-based fuel.
Triflic acid reacts exothermically with alcohols to produce ethers and olefins.
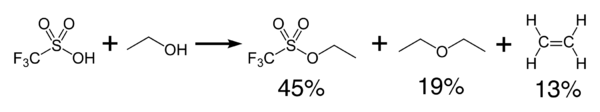
Dehydration gives the acid anhydride, trifluoromethanesulfonic anhydride, (CF3SO2)2O.
Safety
Trifluoromethanesulfonic acid is one of the strongest acids. Contact with skin causes severe burns with delayed tissue destruction. On inhalation it causes fatal spasms, inflammation and edema.[7]
Addition of triflic acid to polar solvents can be dangerously exothermic.
References
- ↑ Howells, R. D., McCown, J. D. (1977). "Trifluoromethanesulfonic Acid and Derivatives". Chemical Reviews 77 (1): 69–92. doi:10.1021/cr60305a005.
- 1 2 Subramanian, L. R.; Martinez, A. G.; Hanack, M.; Prakash, G. K. S.; Hu, J. (2006). "Trifluoromethanesulfonic Acid". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rt246.pub2. ISBN 0-471-93623-5.
- ↑ Siegemund, G.; Schwertfeger, W.; Feiring, A.; Smart, B.; Behr, F.; Vogel, H.; McKusick, B. (2000). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons. doi:10.1002/14356007.a11_349.
- ↑ Haszeldine, R. N.; Kidd, J. M. (1954). "Perfluoroalkyl derivatives of sulphur. Part I. Trifluoromethanesulphonic acid". Journal of the Chemical Society 1954: 4228–4232. doi:10.1039/JR9540004228.
- ↑ Raamat, E.; Kaupmees, K.; Ovsjannikov, G.; Trummal, A.; Kütt, A.; Saame, J.; Koppel, I.; Kaljurand, I.; Lipping, L.; Rodima, T.; Pihl, V.; Koppel, I. A.; Leito, I. "Acidities of strong neutral Brønsted acids in different media." J. Phys. Org. Chem. 2013, 26, 162-170. doi:10.1002/poc.2946
- ↑ Dixon, N. E.; Lawrance, G. A.; Lay, P. A.; Sargeson, A. M.; Taube, H. (1990). "Trifluoromethanesulfonates and trifluoromethanesulfonato-O complexes". Inorganic Syntheses 28: 70–76. doi:10.1002/9780470132593.ch16. ISBN 978-0-470-13259-3.
- ↑ "Trifluoromethanesulfonic acid MSDS". ChemCAS.