Trifluoromethanesulfonate
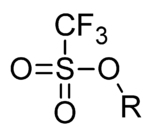

Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3−. The triflate group is often represented by -OTf, as opposed to -Tf (triflyl). For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.
The triflate anion, CF3SO3− is an extremely stable polyatomic ion, being the conjugate base of triflic acid (CF3SO3H), one of the strongest acids known. It is defined as a superacid, because it is more acidic than pure sulfuric acid.
Applications
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which causes the negative charge to be spread over the three oxygen atoms and the sulfur atom. An additional stabilization is achieved by the trifluoromethyl group as a strong electron-withdrawing group.
Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides.
Lithium triflates are used in some lithium ion batteries as a component of the electrolyte.
A mild triflating reagent is phenyl triflimide or N,N-bis(trifluoromethylsulfonyl)aniline, where the by-product is [CF3SO2N-Ph]−.

Triflate salts
Triflate salts are thermally very stable with melting points up to 350 °C for sodium, boron and silver salts especially in water-free form. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate, or from reacting barium triflate with metal sulfates in water:[1]
- MCln + n HOTf → M(OTf)n + n HCl
- MCln + n AgOTf → M(OTf)n + n AgCl ↓
- M(SO4)n + n Ba(OTf)2 → M(OTf)2n + n BaSO4 ↓
Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable in water such as aluminium chloride. Especially useful are the lanthanide triflates of the type Ln(OTf)3 (where Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y). Another popular catalyst scandium triflate is used in such reactions as aldol reactions and Diels-Alder reactions. An example is the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% chemical yield.[2] The corresponding reaction with the yttrium salt fails:
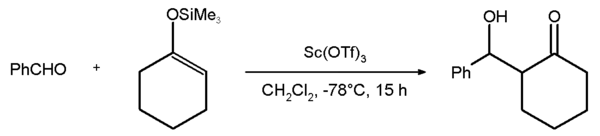
Triflate is a commonly used counterion for organometallic complexes.
See also
References
- ↑ Dixon, N. E.; Lawrance, G. A.; Lay, P. A.; Sargeson, A. M.; Taube, H. (1990). "Trifluoromethanesulfonates and Trifluoromethanesulfonato-O Complexes". Inorganic Syntheses 28: 70–76. doi:10.1002/9780470132593.ch16.
- ↑ Kobayashi, S. "Scandium Triflate in Organic Synthesis". European Journal of Organic Chemistry 1999 (1): 15–27. doi:10.1002/(SICI)1099-0690(199901)1999:1<15::AID-EJOC15>3.0.CO;2-B.