Treatment and prognosis of renal cell carcinoma
The two steps that follow a diagnosis of renal cell carcinoma (RCC) are treatment and prognosis. Traditional cancer treatments such as radiation therapy and chemotherapy are not as effective with this type of cancer, so these are not often used in metastatic renal cell carcinoma treatment. Some cases of carcinoma do respond well to immunotherapy. Medications that work for this kind of treatment include sunitinib, bevacizumab, interferon-alpha, and sorafenib.[1] The most recommended treatment for renal cell cancer is nephrectomy or partial nephrecomty, surgical removal of all or part of the kidney. This may include some of the surrounding organs or tissues or lymph nodes. Chemotherapy, on the other hand, is generally not effective for treating this carcinoma. The drug Interleukin-2 (IL-2), have helped some patients allowing the immune system to kill the cancer cells, although it has proven to be toxic in many cases.[2] Percutaneous and image-guided therapies are an option for people who are not good candidates for a surgical procedure. Survival rates are often used by doctors as a standard way of discussing a person's outlook. The 5-year survival rate defines the percentage of patients who live at least 5 years after renal cell cancer is diagnosed.
Treatment
If cancer is only in the kidneys, which is about 60% of cases, it can be cured roughly 90% of the time with surgery. If it has spread outside of the kidneys, often into the lymph nodes, the lungs or the main vein of the kidney, then multiple therapies are used including surgery and medications. RCC is resistant to chemotherapy and radiotherapy in most cases, but does respond well to immunotherapy with interleukin-2 or interferon-alpha, biologic, or targeted therapy. In early stage cases, cryotherapy and surgery are the preferred options.
Watchful waiting
Small renal tumors (< 4 cm) are treated increasingly by way of partial nephrectomy when possible.[3][4][5] Most of these small renal masses manifest indolent biological behavior with excellent prognosis.[6]
Surgery
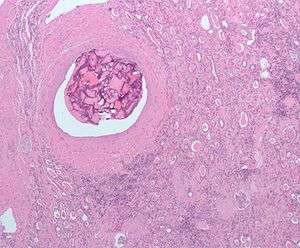
Surgical removal of all or part of the kidney (nephrectomy) is recommended.[7] This may include removal of the adrenal gland, retroperitoneal lymph nodes, and possibly tissues involved by direct extension (invasion) of the tumor into the surrounding tissues. In cases where the tumor has spread into the renal vein, inferior vena cava, and possibly the right atrium, this portion of the tumor can be surgically removed, as well. In cases of known metastases, surgical resection of the kidney ("cytoreductive nephrectomy") may improve survival,[8] as well as resection of a solitary metastatic lesion. Kidneys are sometimes embolized prior to surgery to minimize blood loss[9] (see image).
Surgery is increasingly performed via laparoscopic techniques. These have the advantage of being less of a burden for the patient and the disease-free survival is comparable to that of open surgery.[7] For small exophytic lesions that do not extensively involve the major vessels or urinary collecting system, a partial nephrectomy (also referred to as "nephron sparing surgery") can be performed. This may involve temporarily stopping blood flow to the kidney while the mass is removed as well as renal cooling with an ice slush. Mannitol can also be administered to help limit damage to the kidney. This is usually done through an open incision although smaller lesions can be done laparoscopically with or without robotic assistance.
Laparoscopic cryotherapy can also be done on smaller lesions. Typically a biopsy is taken at the time of treatment. Intraoperative ultrasound may be used to help guide placement of the freezing probes. Two freeze/thaw cycles are then performed to kill the tumor cells. As the tumor is not removed followup is more complicated (see below) and overall disease free rates are not as good as those obtained with surgical removal.
Percutaneous therapies
Percutaneous, image-guided therapies, usually managed by radiologists, are being offered to patients with localized tumor, but who are not good candidates for a surgical procedure. This sort of procedure involves placing a probe through the skin and into the tumor using real-time imaging of both the probe tip and the tumor by computed tomography, ultrasound, or even magnetic resonance imaging guidance, and then destroying the tumor with heat (radiofrequency ablation) or cold (cryotherapy). These modalities are at a disadvantage compared to traditional surgery in that pathologic confirmation of complete tumor destruction is not possible. Therefore, long-term follow-up is crucial to assess completeness of tumour ablation.[10][11]
Medications
RCC "elicits an immune response, which occasionally results in dramatic spontaneous remissions." This has encouraged a strategy of using immunomodulating therapies, such as cancer vaccines and interleukin-2 (IL-2), to reproduce this response. IL-2 has produced "durable remissions" in a small number of patients, but with substantial toxicity. Another strategy is to restore the function of the VHL gene, which is to destroy proteins that promote inappropriate vascularization. Bevacizumab, an antibody to VEGF, has significantly prolonged time to progression, but phase 3 trials have not been published. Sunitinib (Sutent), sorafenib (Nexavar), and temsirolimus, which are small-molecule inhibitors of proteins, have been approved by the U.S. F.D.A.[12]
Treatment with tyrosine kinase inhibitors including nexavar, pazopanib, and rapamycin have shown promise in improving the prognosis for advanced RCC since 2004.
Sorafenib, a protein kinase inhibitor, was FDA approved in December 2005 for treatment of advanced renal cell cancer.
A month later, Sunitinib was approved as well. Sunitinib—an oral, small-molecule, multi-targeted (RTK) inhibitor—and sorafenib both interfere with tumor growth by inhibiting angiogenesis as well as tumor cell proliferation. Sunitinib appears to offer greater potency against advanced RCC, perhaps because it inhibits more receptors than sorafenib. However, these agents have not been directly compared against one another in a single trial.
Recently the first Phase III study comparing an RTKI with cytokine therapy was published in the New England Journal of Medicine. This study showed that Sunitinib offered superior efficacy compared with interferon-α. Progression-free survival (primary endpoint) was more than doubled. The benefit for sunitinib was significant across all major patient subgroups, including those with a poor prognosis at baseline. 28% of sunitinib patients had significant tumor shrinkage compared with only 5% of patients who received interferon-α. Although overall survival data are not yet mature, there is a clear trend toward improved survival with sunitinib. Patients receiving sunitinib also reported a significantly better quality of life than those treated with IFNa.[13]
Temsirolimus (CCI-779) is an inhibitor of mTOR kinase (mammalian target of rapamycin) that was shown to prolong overall survival vs. interferon-α in patients with previously untreated metastatic renal cell carcinoma with three or more poor prognostic features. The results of this Phase III randomized study were presented at the 2006 annual meeting of the American Society of Clinical Oncology (www.ASCO.org).
Date of Approval: March 30, 2009 Company: Novartis AG Treatment for: Renal Cell Carcinoma Afinitor (everolimus) is an oral once-daily inhibitor of mTOR indicated for the treatment of patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib. Afinitor approved in US as first treatment for patients with advanced kidney cancer after failure of either sunitinib or sorafenib - March 30, 2009
Chemotherapy
Most of the currently available cytostatics are ineffective for the treatment of RCC. Their use can not be recommended for the treatment of patients with metastasized RCC,as response rates are very low,often just 5-15%,and most responses are short lived.[9] The use of Tyrosine Kinase (TK) inhibitors, such as Sunitinib and Sorafenib, and Temsirolimus are described in a different section
Vaccine
Cancer vaccines, such as TroVax, have shown promising results in phase 2 trials for treatment of renal cell carcinoma.[14] However, issues of tumor immunosuppression and lack of identified tumor-associated antigens must be addressed before vaccine therapy can be applied successfully in advanced renal cell cancer.[15]
Prognosis
The five-year survival rate is around 90-95% for tumors less than 4 cm. For larger tumors confined to the kidney without venous invasion, survival is still relatively good at 80-85%. For tumors that extend through the renal capsule and out of the local fascial investments, the survivability reduces to near 60%. If it has metastasized to the lymph nodes, the 5-year survival is around 5% to 15%. If it has spread metastatically to other organs, the 5-year survival rate is less than 5%.
The essential factors for a patient's prognosis are the stage of renal cell cancer, the type of treatment received, histological grade of the tumor and the individual’s overall condition. The lower the stage at the time of treatment, the better the prognosis. Tumors confined to the kidney have the most possibilities to heal. For instance, in patients whose disease is limited to the kidney, only 20-30% develop metastatic disease after nephrectomy.[16] Survival rates are often used by doctors as a standard way of discussing a person's outlook. The 5-year survival rate defines the percentage of patients who live at least 5 years after renal cell cancer is diagnosed. Although survival rates are based on previous outcomes of large numbers of patients who had renal cell cancer, they cannot predict what will happen in any particular individual's case. Doctors can tell how these statistics may apply to each patient as they are familiar with the aspects of the person's particular situation. The following numbers are based on patients first diagnosed in 2001 and 2002 by the National Cancer Data Base:[17]
| Stage | Description | 5 Year Survival Rate |
|---|---|---|
| I | Confined to the kidney | 81% |
| II | Extend through the renal capsule, confined to Gerota's Fascia | 74% |
| III | Include the renal vein, or the hilar lymph nodes | 53% |
| IV | Includes tumors that are invasive to adjacent organs(except the adrenal glands), or distant metastases | 8% |
Investigation from the Department of Urology of the University of California- Los Angeles, have found in 2001 several differences between patients with cases of incidentally diagnosed renal cell cancer (no symptoms), and those diagnosed after presenting symptoms of renal cell carcinoma or metastasis. The study was conducted with the records of 633 patients at their institution between 1987 and 1994. 95 (15%) were treated for incidentally discovered renal cell cancer and 538 (85%) presented symptoms when diagnosed with the tumor. The 5 year survival rate was higher for incidental than for symptomatic tumors: 85.3% versus 62.5%. Besides, incidental lesions were significantly lower stage than those that cause symptoms, since 62.1% patients with incidental renal cell carcinoma were observed with Stage I lesions, against 23% were found with symptomatic renal cell carcinoma. This took researchers to hypothesize that incidental tumors are of lower stage and grade, and its less aggressive lesions lead to better patient survival and decreased recurrence. Therefore, the detection of RCC before symptoms enables treatment of less harming tumors and provides a better prognosis for the patient.[18]
However, larger tumors can spread to the lungs, liver, bones or elsewhere. Once the tumor has spread, the patient's length of survival decreases significantly. Despite of this, some people have the renal cell cancer detected before they have symptoms (incidentally) because of the CT scan (Computed Tomography Imaging) or ultrasound. Staging is the most important factor in the outcome of renal cell cancer. Histological grade is related to the aggressiveness of the cancer, and it is classified in 4 grades, with 1 having the best prognosis (5 year survival over 89%), and 4 with the worst prognosis (46% of 5 year survival). In terms of the patient's clinical condition, factors as their general health and fitness or the severity of their symptoms have quite a big impact on their survival rates. For instance, younger people (among 20–40 years old) have a better outcome despite having more symptoms at presentation, possibly due to lower rates spread of cancer to their lymph nodes (stage III).
References
- ↑ Renal Cell Carcinoma Prognosis and Treatment Carcinoma Prognosis. Retrieved on 2010-09-10
- ↑ Renal Cell Carcinoma MedlinePlus Medical Encyclopedia. Retrieved on 2010-09-10
- ↑ Novick AC (September 1998). "Nephron-sparing surgery for renal cell carcinoma". Br J Urol 82 (3): 321–4. doi:10.1046/j.1464-410X.1998.00751.x. PMID 9772865.
- ↑ Herr HW (January 1999). "Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup". J. Urol. 161 (1): 33–4; discussion 34–5. doi:10.1016/S0022-5347(01)62052-4. PMID 10037361.
- ↑ Van Poppel H, Bamelis B, Oyen R, Baert L (September 1998). "Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control". J. Urol. 160 (3 Pt 1): 674–8. doi:10.1016/S0022-5347(01)62751-4. PMID 9720519.
- ↑ Mattar K, Jewett MA (January 2008). "Watchful waiting for small renal masses". Curr Urol Rep 9 (1): 22–5. doi:10.1007/s11934-008-0006-3. PMID 18366970.
- 1 2 Rini BI, Rathmell WK, Godley P (May 2008). "Renal cell carcinoma". Curr Opin Oncol 20 (3): 300–6. doi:10.1097/CCO.0b013e3282f9782b. PMID 18391630.
- ↑ Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED (March 2004). "Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis". J Urol. 171 (3): 1071–6. doi:10.1097/01.ju.0000110610.61545.ae. PMID 14767273.
- 1 2 Mulders PF, Brouwers AH, Hulsbergen-van der Kaa CA, van Lin EN, Osanto S, de Mulder PH (February 2008). "[Guideline 'Renal cell carcinoma']". Ned Tijdschr Geneeskd (in Dutch and Flemish) 152 (7): 376–80. PMID 18380384.
- ↑ Mogami T, Harada J, Kishimoto K, Sumida S (April 2007). "Percutaneous MR-guided cryoablation for malignancies, with a focus on renal cell carcinoma". Int. J. Clin. Oncol. 12 (2): 79–84. doi:10.1007/s10147-006-0654-6. PMID 17443274.
- ↑ Boss A, Clasen S, Kuczyk M, Schick F, Pereira PL (March 2007). "Image-guided radiofrequency ablation of renal cell carcinoma". Eur Radiol 17 (3): 725–33. doi:10.1007/s00330-006-0415-y. PMID 17021704.
- ↑ Michaelson MD, Iliopoulos O, McDermott DF, McGovern FJ, Harisinghani MG, Oliva E (May 2008). "Case records of the Massachusetts General Hospital. Case 17-2008. A 63-year-old man with metastatic renal-cell carcinoma". N Engl J Med. 358 (22): 2389–96. doi:10.1056/NEJMcpc0802449. PMID 18509125.
- ↑ Motzer RJ, et al. (2007). "Sunitinib versus interferon alfa in metastatic renal-cell carcinoma". N Engl J Med 356 (2): 115–124. doi:10.1056/NEJMoa065044. PMID 17215529.
- ↑ "Vaccine for kidney and bowel cancers 'within three years'". Daily Mail (London). 2006-11-13.
- ↑ Amato RJ (September 2008). "Vaccine therapy for renal cancer". Expert Rev Vaccines 7 (7): 925–35. doi:10.1586/14760584.7.7.925. PMID 18767943.
- ↑ Renal Cancer Causes, Symptoms, Treatment. eMedicine Health. Retrieved on 2010-09-10
- ↑ Kidney Cancer (Adult) – Renal Cell Carcinoma American Cancer Society. Retrieved on 2010-09-10
- ↑ Renal Cell Carcinoma: prognostic significance of incidentally detected tumors Kidney Cancer (Renal Cell Carcinoma; RCC). U.S National Library of Medicine. National Institutes of Health. Retrieved on 2010-09-10